Phase III EMERALD-1 trial shows Imfinzi plus bevacizumab improves progression-free survival in embolisation-eligible advanced liver cancer
Encouraging top-tier findings from the Phase III EMERALD-1 trial revealed that the combo of AstraZeneca’s Imfinzi (durvalumab) with transarterial chemoembolization and bevacizumab manifested a statistically pertinent and medically significant enhancement in PFS as the prime objective versus TACE alone in patients with hepatocellular carcinoma qualified for embolization. The trial still monitors the secondary endpoint of general survival.
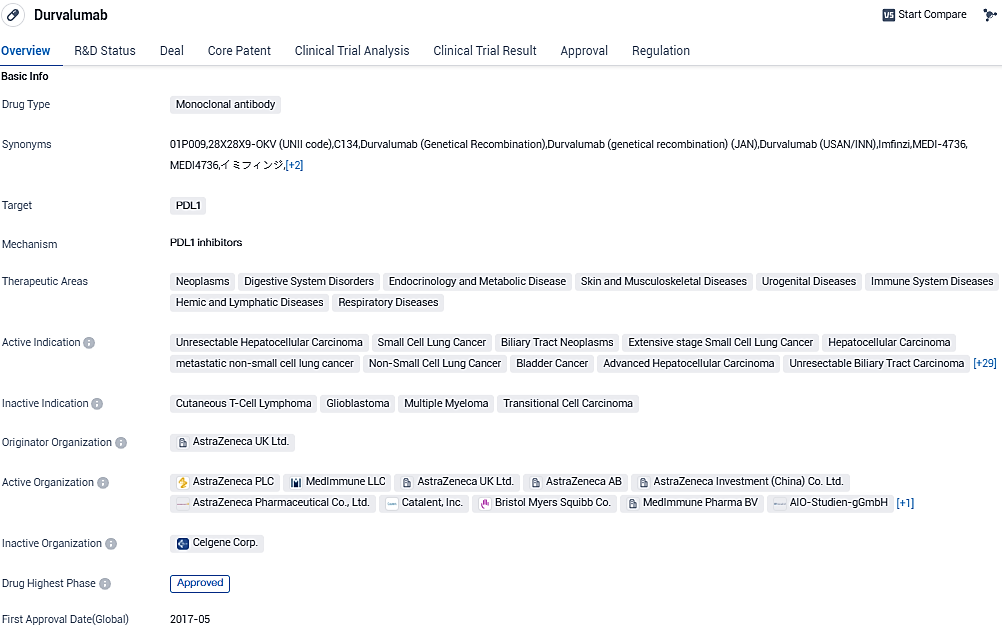
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Hepatocellular carcinoma (HCC) is the most prevalent type of liver cancer and ranks third in cancer fatalities, with roughly 900,000 diagnoses globally each year. A technique called embolisation, which obstructs the tumour's blood flow and can directly administer chemotherapy or radiation therapy to the liver, is suitable for approximately 20-30% of patients.
Despite it being considered the standard treatment in such scenarios, rapid disease escalation or relapse is often experienced by the majority of embolisation recipients. Dr. Riccardo Lencioni, the Director of the Cancer Imaging Program in the Department of Diagnostic and Interventional Radiology at Pisa University Hospital in Pisa, Italy, commented: "The outcomes in utilising durvalumab plus bevacizumab could revolutionize the approach towards treating this challenging disease with a grim prognosis by demonstrating for the first time that incorporating an immunotherapy cocktail to TACE significantly extends progression-free survival."
AstraZeneca's Executive Vice President of Oncology R&D, Susan Galbraith, said: “These encouraging findings for Imfinzi-based therapy in EMERALD-1 could potentially extend the benefits of immunotherapy to patients in earlier stages of liver cancer. We aim to bring this innovative treatment to patients and are eager to engage in talks with regulatory bodies about these data and observe the maturation of survival data over time."
AstraZeneca is conducting an expansive clinical research program to further investigate Imfinzi in various gastrointestinal cancer scenarios, which includes an adjuvant HCC trial combining Imfinzi with bevacizumab (EMERALD-2), and a study involving tremelimumab, lenvatinib, and TACE for embolisation-eligible HCC (EMERALD-3).
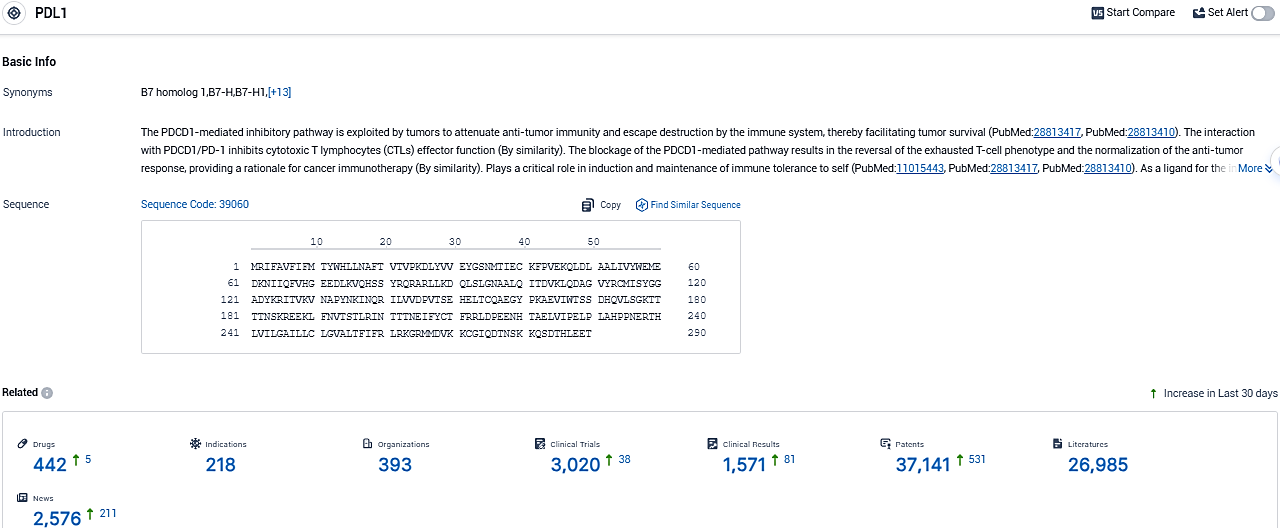
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 16, 2023, there are 442 investigational drugs for the PDL1 and integrin target, including 218 indications, 393 R&D institutions involved, with related clinical trials reaching 3020, and as many as 37141 patents.
Imfinzi (durvalumab) is a human monoclonal antibody that binds to the PD-L1 protein and blocks the interaction of PD-L1 with the PD-1 and CD80 proteins. Imfinzi is approved in combination with chemotherapy in locally advanced or metastatic biliary tract cancer and in combination with Imjudo (tremelimumab) in unresectable HCC in the US, EU, Japan and many other countries based on the TOPAZ-1 and HIMALAYA Phase III trials, respectively.