Pierre Fabre and Scorpion Therapeutics Initiate Phase I/II Trial with First Patient Dosed Using PFL-241/STX-241
Pierre Fabre Laboratories, which operates globally in the field of oncology, together with Scorpion Therapeutics, Inc., a leading clinical-stage oncology firm, have recently reported that they have started dosing the first participant in a Phase I/II clinical trial. This initial trial involves dose-escalation, dose-optimization, and dose-expansion stages in human subjects. The study is targeting PFL-241/STX-241, an advanced and orally administered tyrosine kinase inhibitor (TKI) with high selectivity. The focus of the investigation is on mutations in the epidermal growth factor receptor (EGFR), specifically Exon 19 or 21 mutations along with the C797S mutation, which is a recognized resistance mechanism to third-generation EGFR inhibitors.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The PFL-241/STX-241 Phase I/II trial is an open-label, multi-center study aimed at evaluating the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD), and initial clinical effectiveness of PFL-241/STX-241 when used alone in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) carrying EGFR Exon 19 or 21 mutations alongside the C797S mutation.
NSCLC is the most prevalent type of lung cancer, with EGFR mutations being a major contributor to the disease, identified in up to 38% of tumors based on location1,2,3.
"We are excited to embark on the clinical testing of PFL-241/STX-241, our mutant-selective fourth-generation EGFR inhibitor, which possesses distinct characteristics we believe may establish it as a leading therapeutic choice for patients who develop resistance to current targeted treatments," stated Francesco Hofmann, the Head of Research and Development for Medical Care at Pierre Fabre Laboratories. "Starting this clinical trial reflects our team's dedication and effective collaboration with Scorpion Therapeutics, and we anticipate showing how patients might gain from this targeted therapy."
PFL-241/STX-241 is an oral medication developed to specifically target the C797S resistance mutation that co-exists with EGFR exon 19 deletion or exon 21 mutation ("double mutant"). These "double mutants" are becoming known as a direct resistance mechanism in a group of NSCLC patients. Recent findings indicate that the C797S mutation surfaces in about 12.5%4-8 of patients treated with third-generation EGFR inhibitors. Currently, no approved treatments are available for patients with "double mutant" EGFR NSCLC.
"The launch of the second clinical trial in partnership with Pierre Fabre Laboratories marks a significant step as we collaboratively advance our next-generation EGFR inhibitors for challenging NSCLC cases at a global level," expressed Mark Chao, M.D., Ph.D., Chief Medical Officer of Scorpion. "PFL-241/STX-241 represents a new, CNS-penetrant, and highly powerful and selective treatment option for individuals who develop 'double mutant' conditions for which there are no approved treatments currently, and it's an honor to partner with Pierre Fabre Laboratories, a firm committed to delivering innovative therapies to this underserved patient group. We are eager to demonstrate how PFL-241/STX-241's unique preclinical characteristics can be translated into clinical benefits for patients."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
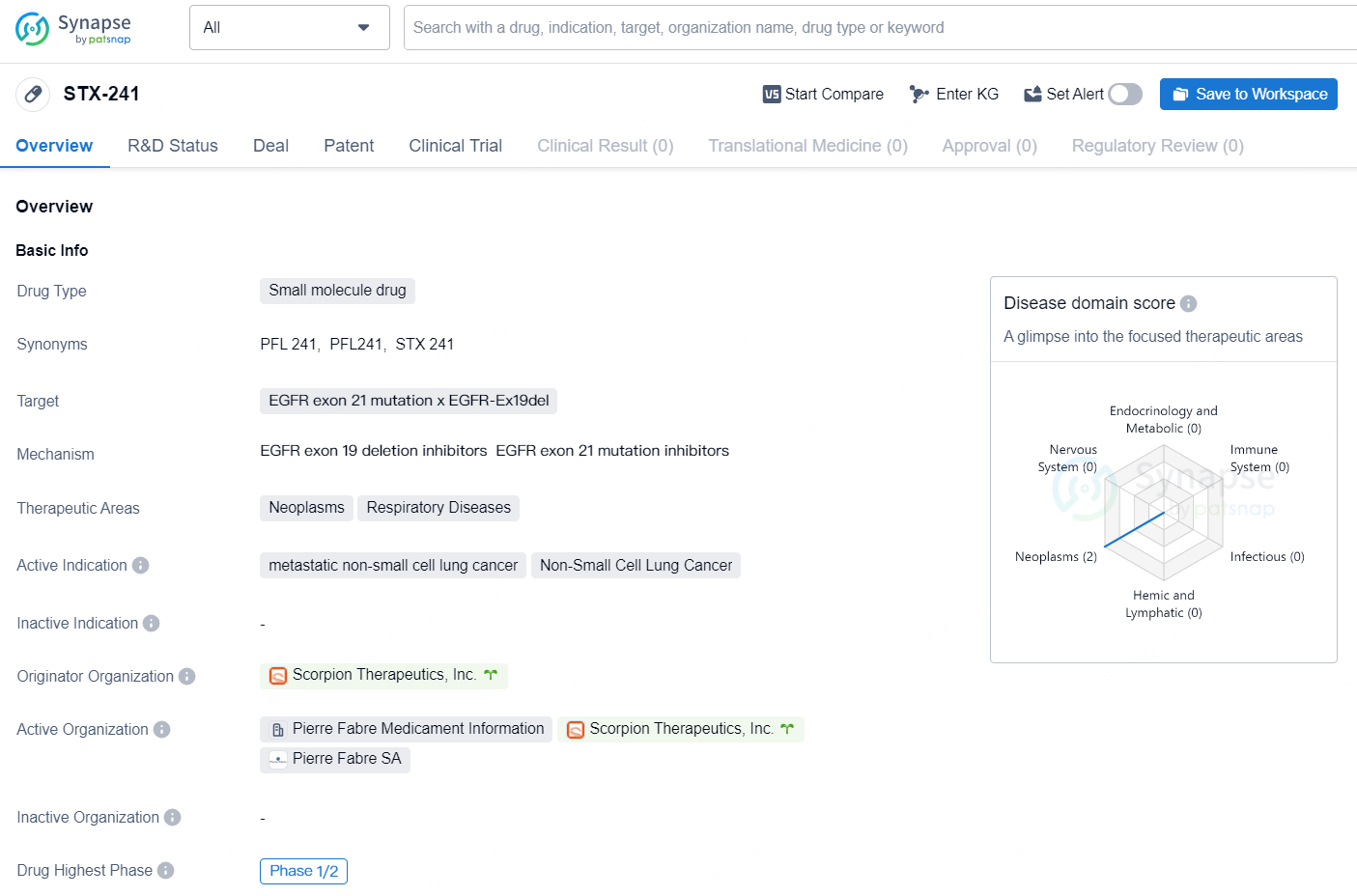
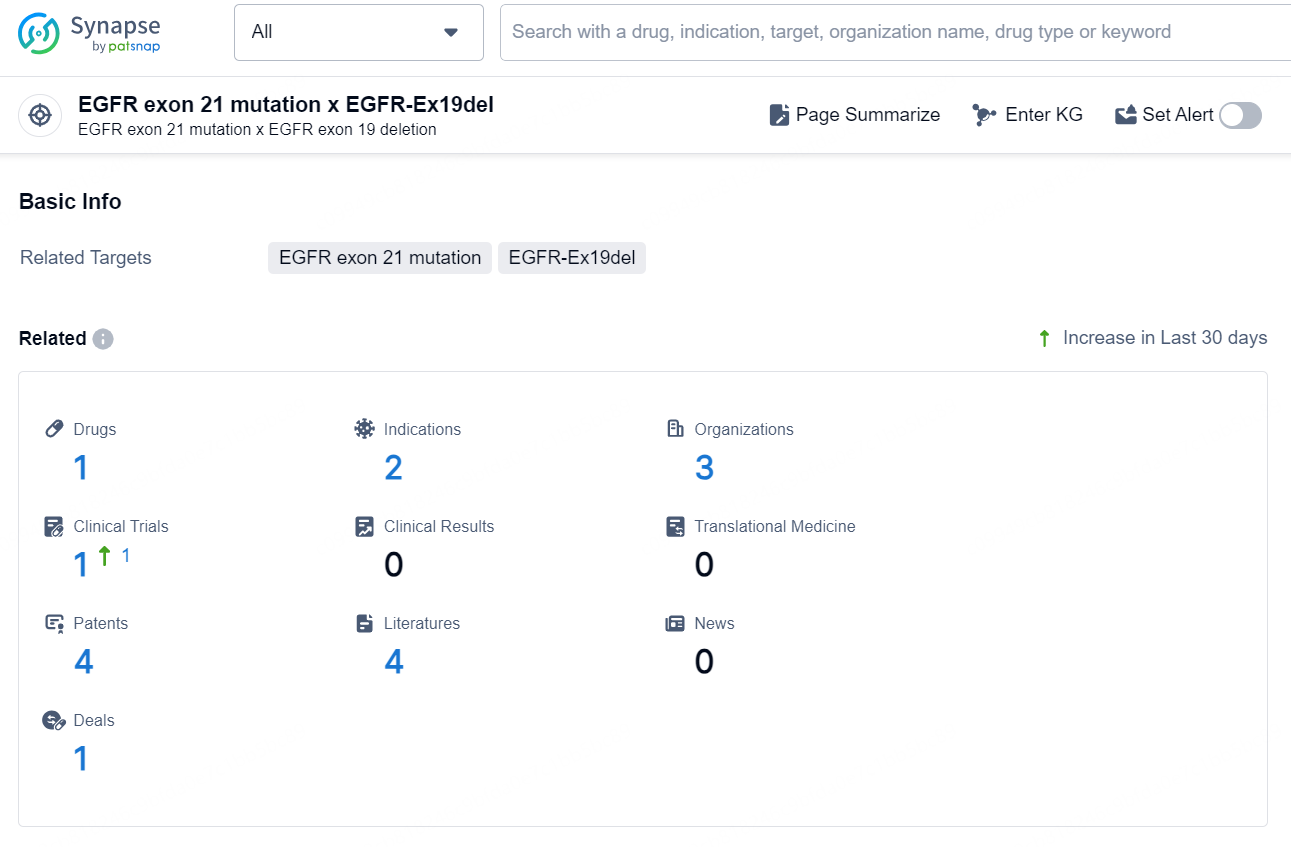
According to the data provided by the Synapse Database, As of October 10, 2024, there are 1 investigational drug for the EGFR exon 21 mutation x EGFR-Ex19del targets, including 5 indications, 1 R&D institution involved, with related clinical trial reaching 1, and as many as 92 patents.
STX-241 is a small molecule drug developed by Scorpion Therapeutics, Inc. The drug targets EGFR exon 21 mutation x EGFR-Ex19del and is indicated for the treatment of metastatic non-small cell lung cancer and Non-Small Cell Lung Cancer. Its therapeutic areas include neoplasms and respiratory diseases. STX-241 has reached the highest phase of clinical development as Phase 1/2 globally and also in China. This indicates that the drug has progressed beyond initial safety testing and is now being studied in a larger group of patients to determine its effectiveness and optimal dosage.