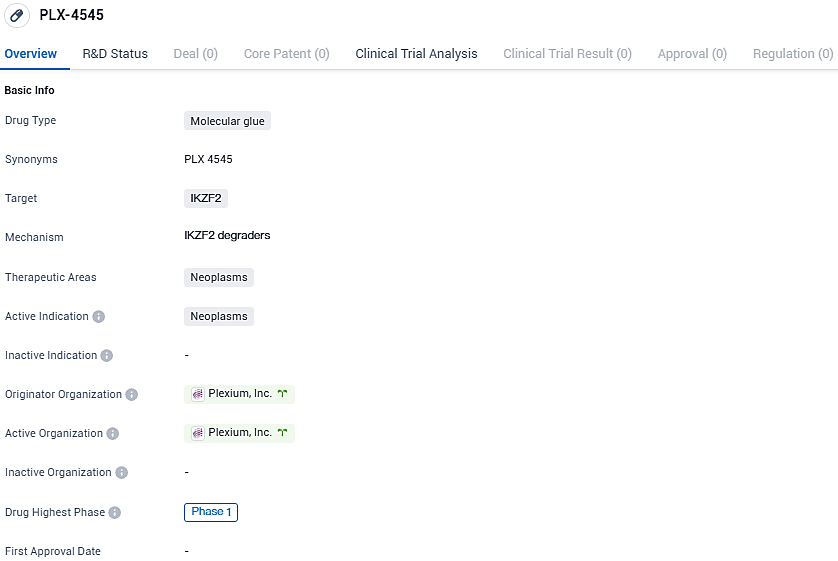
Plexium has begun administering PLX-4545, its targeted IKZF2 transcription factor degrader, in a first-stage clinical trial
Plexium, Inc., an innovative company at the forefront of advancing targeted protein degradation therapies, has announced that initial participants in a Phase 1 clinical trial have received doses of PLX-4545. This orally delivered treatment is a powerful and specific molecular glue degrader designed to target IKZF2, a transcription factor traditionally considered "undruggable" which is alternatively named Helios.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"It is with great enthusiasm that we announce the commencement of dosing in our inaugural Phase 1 trial for PLX-4545, propelling Plexium into the realm of clinical research companies," declared Mike Grey, the Executive Chairman at Plexium. "This pivotal step marks a significant achievement as we transition PLX-4545, a premier small molecule degrader derived from our exclusive drug development platform, into trials involving human participants."
The structured Phase 1 clinical trial, which is randomized, double-blind, placebo-controlled, and includes both single and multiple ascending doses, aims to meticulously evaluate the safety and tolerability of PLX-4545 when given orally. Additionally, it will explore pharmacokinetics and pharmacodynamics to determine an efficacious dose.
"Even though checkpoint inhibitors have shown extensive activity across various types of tumors, a significant number of cancer patients remain non-responsive to these treatments, often due to the presence of immune suppression in the tumor stroma," explained Simon Bailey, Ph.D., the Executive Vice President of Drug Discovery at Plexium.
"Within the realm of immune transcription factors, IKZF2 stands out as an indicator of the highly suppressive regulatory T cells. Our research has confirmed that the targeted and potent degradation of IKZF2 leads to the transformation of regulatory T cells into cells that act more like effector T cells. Moreover, our findings reveal that the oral delivery of PLX-4545 in preclinical cancer models has yielded encouraging results, with even greater effectiveness observed when combined with a checkpoint inhibitor," elaborated Dr. Bailey.
Dr. Bailey further remarked, "The data collected from this preliminary investigation will lay a solid foundation for future clinical trials aimed at assessing PLX-4545's potential in treating cancer patients whose tumors have not responded to checkpoint inhibitors. We are particularly interested in exploring its use both as a stand-alone treatment and in conjunction with checkpoint inhibitors, targeting a critical area of unaddressed medical need."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
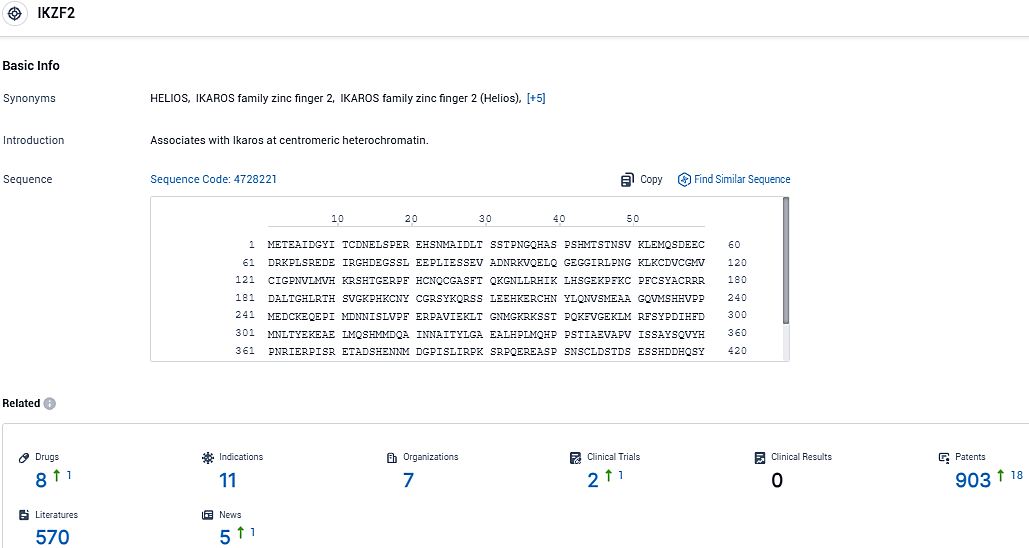
According to the data provided by the Synapse Database, As of December 19, 2023, there are 8 investigational drugs for the IKZF2 target, including 11 indications, 7 R&D institutions involved, with related clinical trials reaching 2, and as many as 903 patents.
PLX-4545 is a potent, selective and orally bioavailable molecular glue degrader of IKZF2, designed to destabilize highly suppressive regulatory T cells. PLX-4545 delivers rapid, deep and selective degradation of IKZF2 resulting in destabilization of Tregs in vitro and in vivo. In preclinical studies, PLX-4545 has demonstrated single agent anti-tumor activity in vivo comparable to pembrolizumab and increased efficacy in combination.