Deciphering RET Inhibitors and Keeping Up with Their Recent Developments
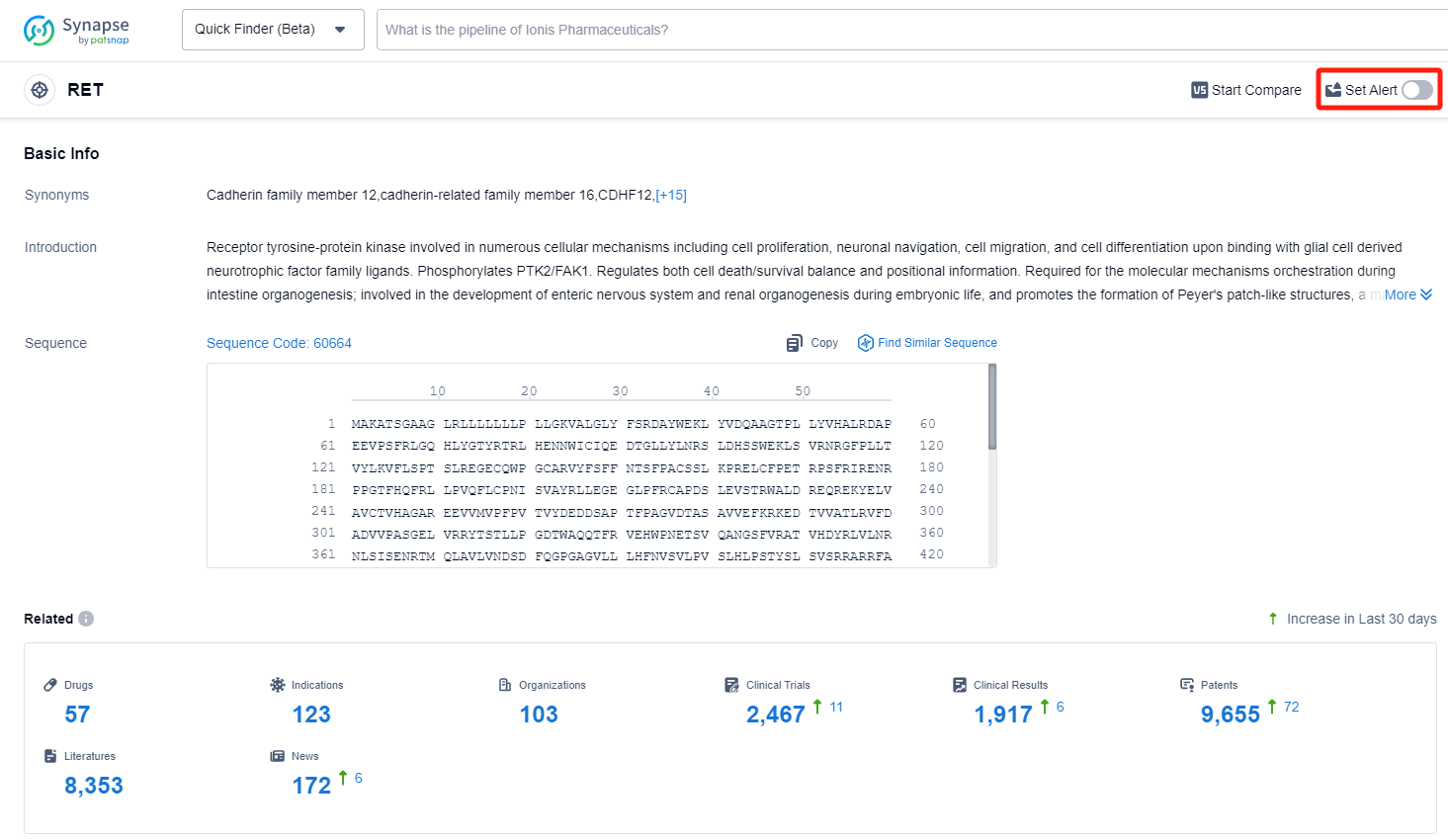
RET, or the Rearranged during Transfection gene, plays a crucial role in the human body. It encodes a receptor tyrosine kinase that is essential for the development and maintenance of various tissues and organs. RET is primarily involved in the regulation of cell growth, survival, and differentiation. It acts as a signaling molecule, transmitting signals from the extracellular environment to the cell's interior, thereby influencing cellular processes. Dysregulation or mutations in RET can lead to various diseases, including cancer and developmental disorders. Understanding the role of RET is vital for the development of targeted therapies and diagnostic tools in the pharmaceutical industry.
As an oncogene, RET is located on the long arm of chromosome 10 and encodes a receptor tyrosine kinase. The pathogenesis of RET-related tumors mainly involves changes in the RET gene and abnormal expression of the wild-type RET gene. There are two forms of RET gene changes: RET fusion and RET mutation. The incidence of RET mutations is much higher than that of RET fusion, but most RET mutations do not have clinical significance, while RET fusion changes are related to the occurrence and development of various cancers. With the deepening of research on RET gene mutations, RET is becoming a target for precision treatment of various tumors.
The current competitive landscape for target RET is characterized by the involvement of multiple pharmaceutical companies, with Roche Holding AG and Bayer AG leading in terms of the highest stage of development. These companies are actively progressing their drugs through clinical trials, indicating a strong focus on bringing potential treatments to market.
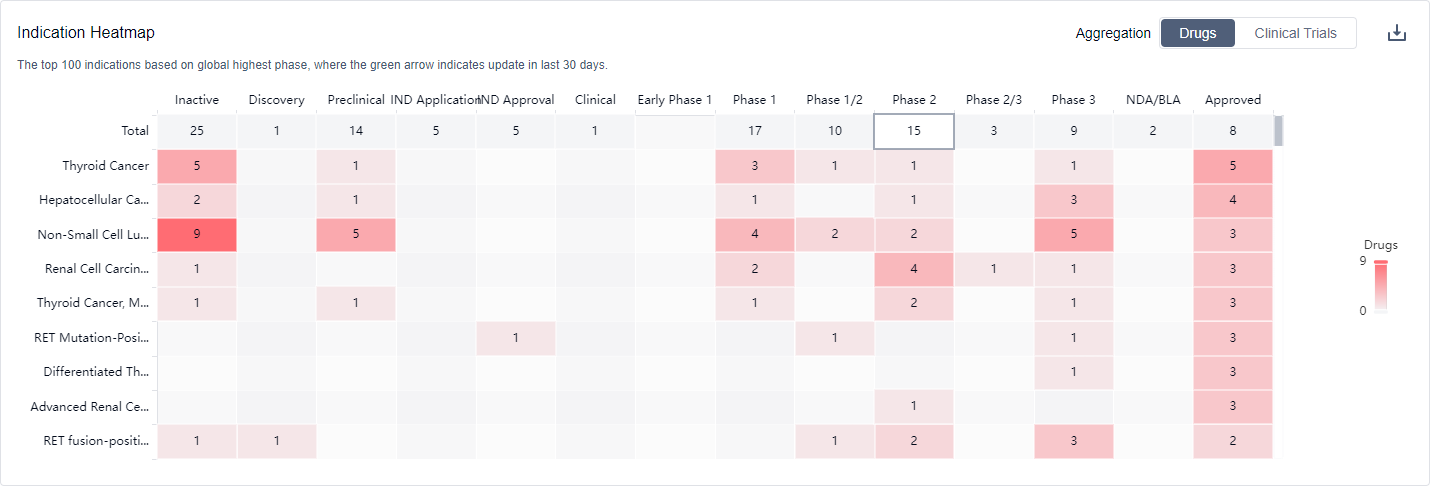
The indications for drugs targeting RET cover a wide range of cancers and related conditions, highlighting the potential of RET-targeted therapies in various disease areas.
The rapid progress of small molecule drugs and the presence of biosimilars suggest intense competition and innovation in the development of RET-targeted therapies.
China has emerged as a significant player in the development of drugs targeting RET, with notable progress in terms of approved drugs and ongoing research.
Overall, the analysis of the current competitive landscape and future development of target RET indicates a promising outlook for the advancement of treatments for RET-related conditions. The involvement of multiple companies, the diversity of indications, and the focus on small molecule drugs and biosimilars contribute to a dynamic and competitive market in the pharmaceutical industry.
How do they work?
RET inhibitors are a type of medication that specifically target and inhibit the activity of the RET (rearranged during transfection) protein. From a biomedical perspective, RET is a receptor tyrosine kinase that plays a crucial role in cell signaling and growth. It is involved in various physiological processes, including the development of the nervous system and the formation of certain tissues and organs.
When the RET protein becomes mutated or overactive, it can contribute to the development and progression of certain diseases, particularly cancers. RET inhibitors work by binding to the RET protein and blocking its activity, thereby inhibiting the abnormal signaling pathways that promote tumor growth and spread.
In the context of cancer treatment, RET inhibitors are being investigated as potential targeted therapies for specific types of cancers that harbor RET gene alterations, such as certain forms of thyroid cancer and non-small cell lung cancer. By selectively inhibiting the RET pathway, these inhibitors aim to disrupt the growth and survival of cancer cells while sparing normal cells.
It is important to note that RET inhibitors are still under investigation and not yet approved for all indications. Clinical trials are ongoing to evaluate their safety, efficacy, and potential side effects. As with any medication, the use of RET inhibitors should be guided by healthcare professionals and tailored to each individual patient's condition.
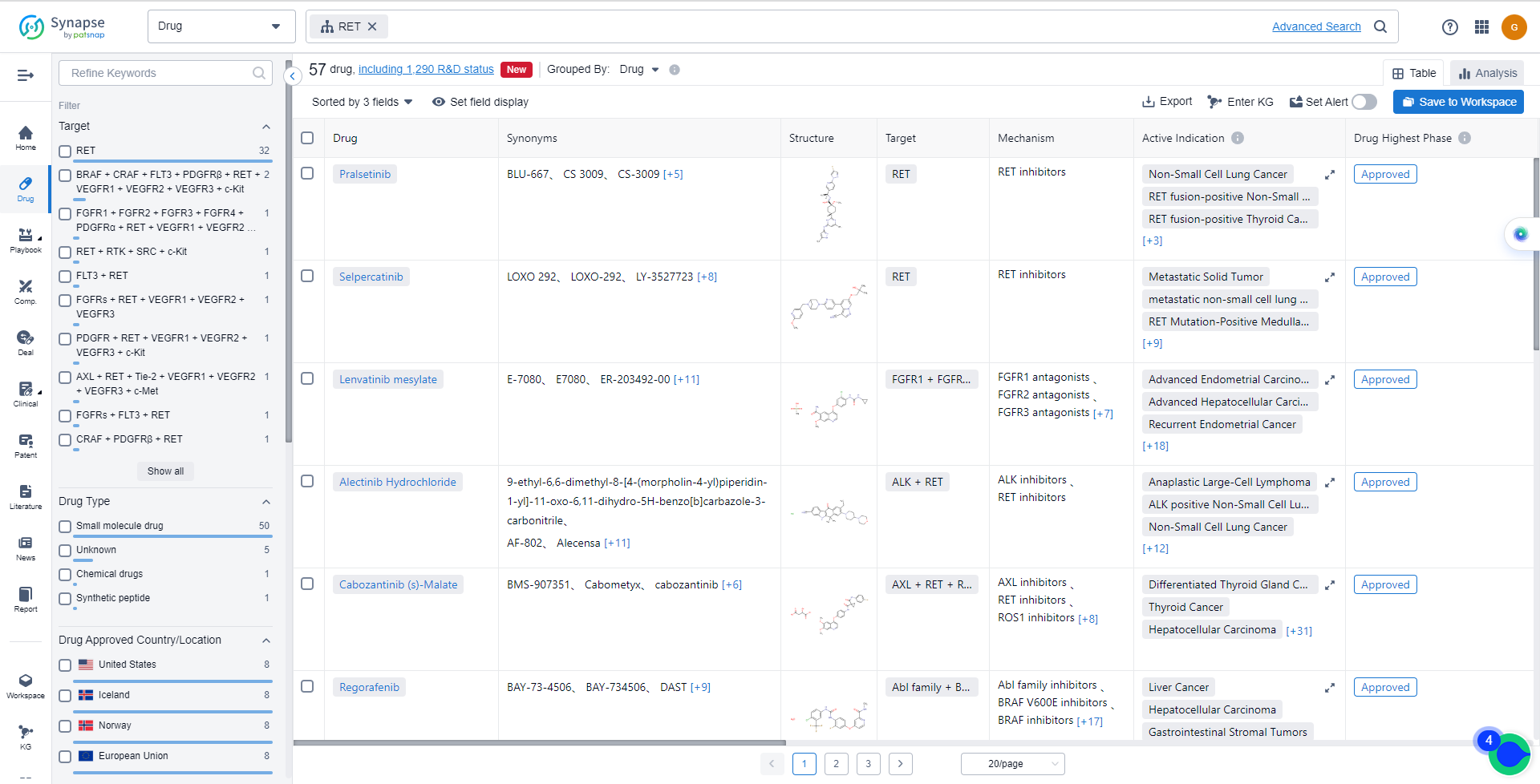
List of RET Inhibitors
The currently marketed RET inhibitors include:
- Pralsetinib
- Selpercatinib
- Lenvatinib mesylate
- Alectinib Hydrochloride
- Cabozantinib (s)-Malate
- Regorafenib
- Vandetanib
- Sorafenib Tosylate
- Sitravatinib
- SY-5007
For more information,please click on the image below.
What are RET inhibitors used for?
RET inhibitors are used for specific types of cancers that harbor RET gene alterations, such as certain forms of thyroid cancer and non-small cell lung cancer. For more information, please click on the image below to log in and search.
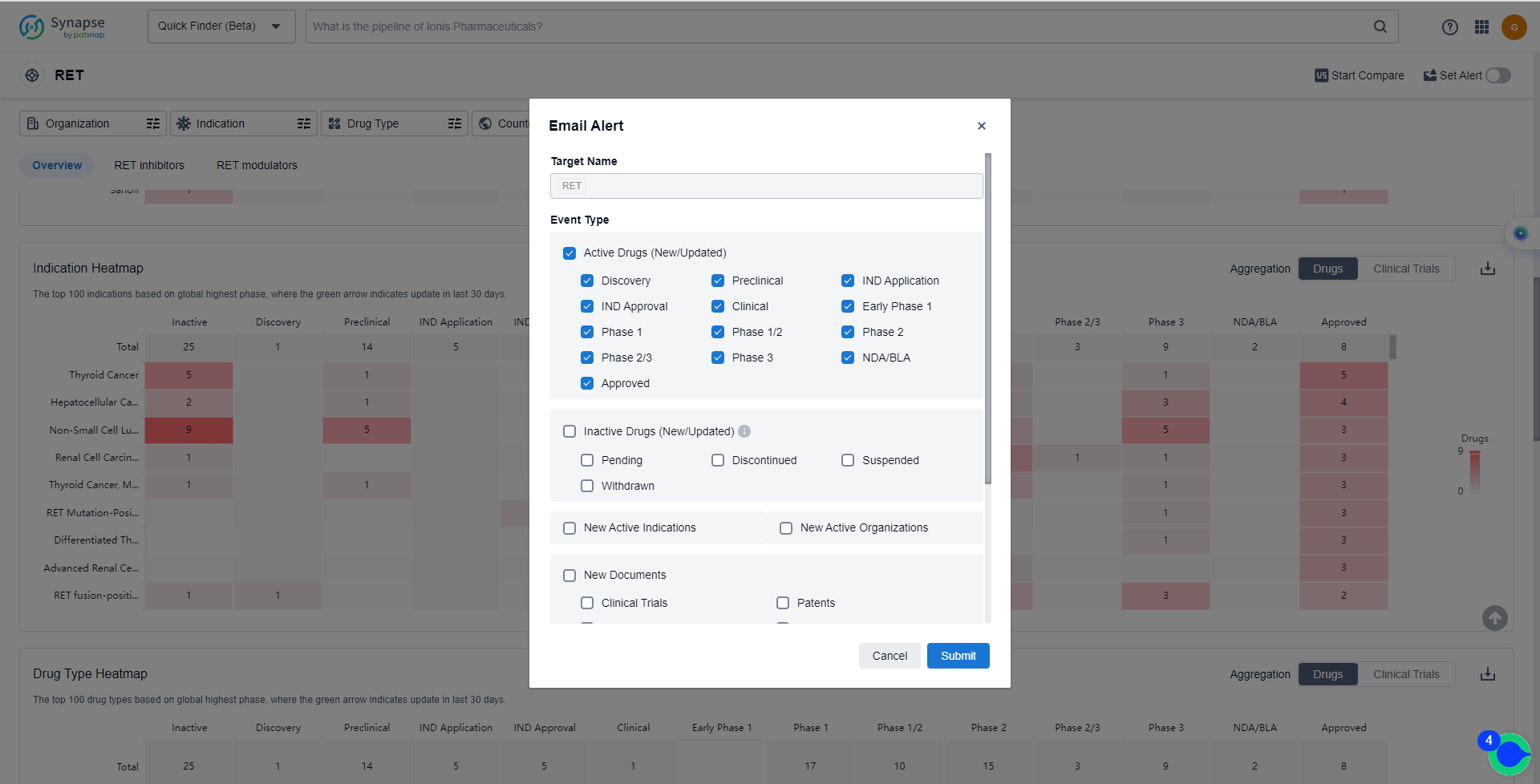
How to obtain the latest development progress of RET inhibitors?
In the Synapse database, you can keep abreast of the latest research and development advances of RET inhibitors anywhere and anytime, daily or weekly, through the "Set Alert" function. Click on the image below to embark on a brand new journey of drug discovery!