Acepodia Gets US Approval for Innovative Anti-EGFR Cell Therapy Trial, ACE2016
Acepodia, an innovative biotech enterprise at the clinical phase, specializing in creating novel cellular treatments, utilizes its distinct Antibody-Cell Conjugation technology and allogeneic gamma delta 2 T cell platform to combat the unmet needs in oncology treatment. The organization has recently declared that their new therapeutic candidate, ACE2016, which is an allogeneic gamma delta 2 T cell therapy targeted at solid tumors presenting epidermal growth factor receptor (EGFR)-positive malignancies, has received authorization from the U.S. Food and Drug Administration for its investigational new drug application.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Acepodia is authorized to move forward with a Phase 1 clinical study, pioneering its evaluation on humans to assess ACE2016's safety profile, dose tolerance, and pharmacodynamic properties in adult individuals diagnosed with EGFR-positive solid cancers that have progressed locally or spread. The initiation of this clinical study is planned within the next few months, with expectations to administer treatment to the initial participant during the latter half of the year 2024.
"Our achievement in reaching this pivotal junction is instrumental in propelling our lineup of innovative cellular treatments forward and in investigating the efficacy of our unique Antibody-Cell Conjugation approach against solid malignancies, which currently present substantial unaddressed challenges within the realm of cellular therapies," commented Sonny Hsiao, Ph.D., CEO of Acepodia.
Sonny Hsiao further expressed, "Securing our third IND clearance in less than one and a half years is a testament to our team's exceptional capabilities and commitment to rapidly pushing forward with pioneering treatments. As we introduce our third therapeutic candidate into clinical studies, we stand committed to enhancing the cellular therapy landscape, aspiring to provide potent and widely available therapeutic options to patients through an unparalleled methodology."
ACE2016, a γδ2 T cell therapy product available off-the-shelf, is crafted using Acepodia's proprietary ACC technology. Designed to target solid tumors expressing EGFR by employing γδ2 T cells in conjunction with antibodies, ACE2016 zeroes in on tumors influenced by the oncogenic EGFR mutation. By harnessing the synergistic potential of ACC technology alongside Acepodia's proprietary γδ2 T cell innovation, ACE2016 has exhibited significant cytotoxic efficacy against multiple EGFR-positive cancer types in a series of preclinical investigations.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
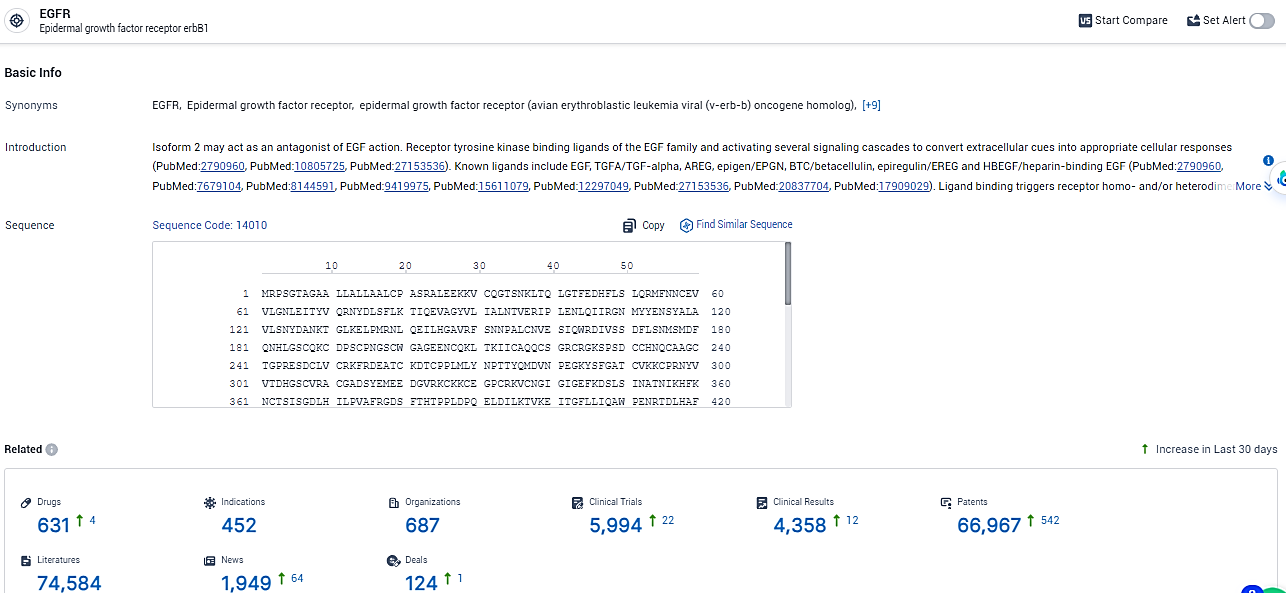
According to the data provided by the Synapse Database, As of February 8, 2024, there are 631 investigational drugs for the EGFR target, including 452 indications, 687 R&D institutions involved, with related clinical trials reaching 5994, and as many as 66967 patents.
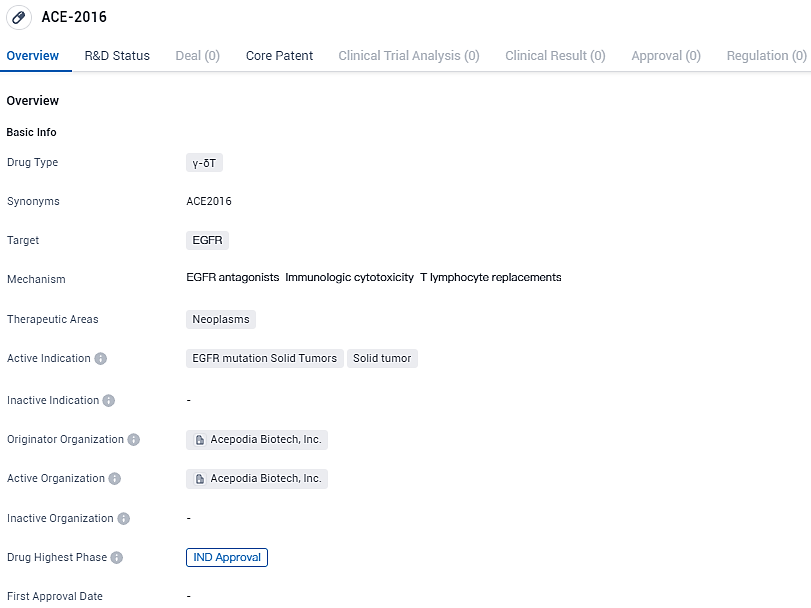
ACE-2016 is a γ-δT drug that targets EGFR and is indicated for the treatment of solid tumors with EGFR mutations. It has received IND Approval, indicating its readiness for clinical trials. The drug holds potential in addressing the therapeutic needs of patients with neoplasms, particularly those with solid tumors driven by EGFR mutations.