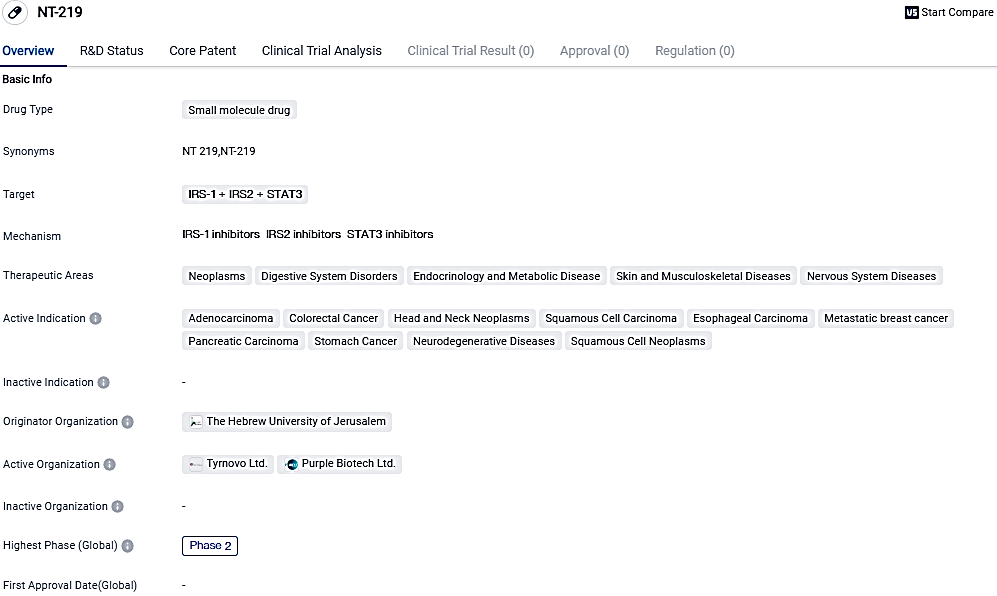
Preliminary Findings from NT219 Phase 1/2 Trial in Head & Neck Cancer Announced by Purple Biotech
Purple Biotech Ltd. shares clinical insights from its dose amplification segment of the Phase 1/2 trial of NT219, a unique dual inhibitor of Insulin receptor substrate 1/2 and Signal Transducer and Activator of Transcription 3 (STAT3). Additionally, the company offers its perception on strategizing the forthcoming clinical development phases for NT219, targeting second-line treatment in patients with recurring and/or metastatic squamous cell carcinoma of the head and neck.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Phase 1/2 experiment is an open-label, dose-escalation and expansion exam aiming to evaluate the safety, pharmacokinetics, pharmacodynamics, and efficacy of NT219--both as a sole treatment option and in combination with Erbitux® (cetuximab). Target populations are patients with Advanced Colorectal Cancer or R/M SCCHN.
The trial involved 49 patients spread across five dosage categories. 27 patients received NT219 as a sole treatment option, and 22 were treated in combination with cetuximab. Both groups were administered the maximum NT219 dosage of 50mg/kg. There were no reported incidents of dose-related toxicity in either group, and NT219 was well received as both a monotherapy and when combined with cetuximab.
Substantially increased drug exposure of NT219 was noted. Furthermore, exposure at the highest dose level of 50mg/kg was within the efficiency range for a human equivalent dose based on predictions from preclinical paradigms. Biopsies from patients showed inhibition of intra-tumoral IRS 1/2 and STAT3--the targets of NT219.
Four R/M SCCHN patients were administered 50mg/kg of NT219 in combination with cetuximab, and this group was available for an efficacy assessment. Two of these patients showed confirmed partial response under response evaluation criteria in solid tumors. Additional findings from the Phase 1/2 study will be disclosed at an upcoming medical gathering.
"These outcomes are inspiring, bearing in mind the low response rate in recurrent/metastatic SCCHN patients in the second/third line when only using cetuximab," noted Ari Rozenberg, M.D, University of Chicago and study Investigator. "I am excited about further exploring NT219's potential and seeing more data in this urgently needing improved treatment setting."
Treatment options for patients who had progressed following treatment with immunotherapy in first line R/M SCCHN are limited, with mostly cetuximab and chemotherapy currently available. The Company is in the process of designing a Phase 2 study of NT219 in combination with cetuximab in 2L R/M SCCHN. Such a study may evaluate NT219 in combination with cetuximab with or without standard of care chemotherapy following progression after immunotherapy in first line treatment.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
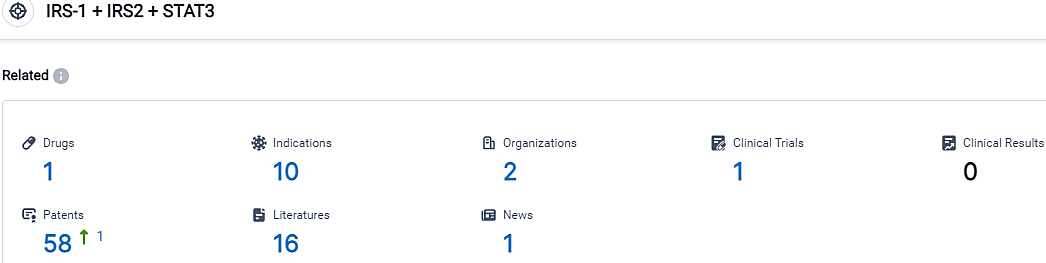
According to the data provided by the Synapse Database, As of October 11, 2023, there are 1 investigational drugs for the IRS-1 and IRS2 and STAT3 target, including 10 indications, 2 R&D institutions involved, with related clinical trials reaching 1,and as many as 58 patents.
NT-219 is a small molecule drug being developed for the treatment of various diseases, with a focus on neoplasms and digestive system disorders. Its unique targeting of IRS-1, IRS2, and STAT3 proteins sets it apart from other drugs in development. While it is still in Phase 2 of clinical development, early results suggest its potential as a novel therapeutic option. Further research and clinical trials will be necessary to determine its ultimate effectiveness and safety.