Promising Phase 1 Outcomes for Trishula’s TTX-030 in Metastatic Pancreatic Cancer
Trishula Therapeutics, Inc., a private biotechnology company in the clinical stages of development, has announced the conclusive results of a Phase 1 clinical trial for TTX-030. This novel anti-CD39 antibody is being evaluated as a potential first-in-class treatment for patients suffering from first-line metastatic pancreatic cancer. The findings were revealed at the 2024 European Society for Medical Oncology (ESMO) Congress held in Barcelona, Spain.
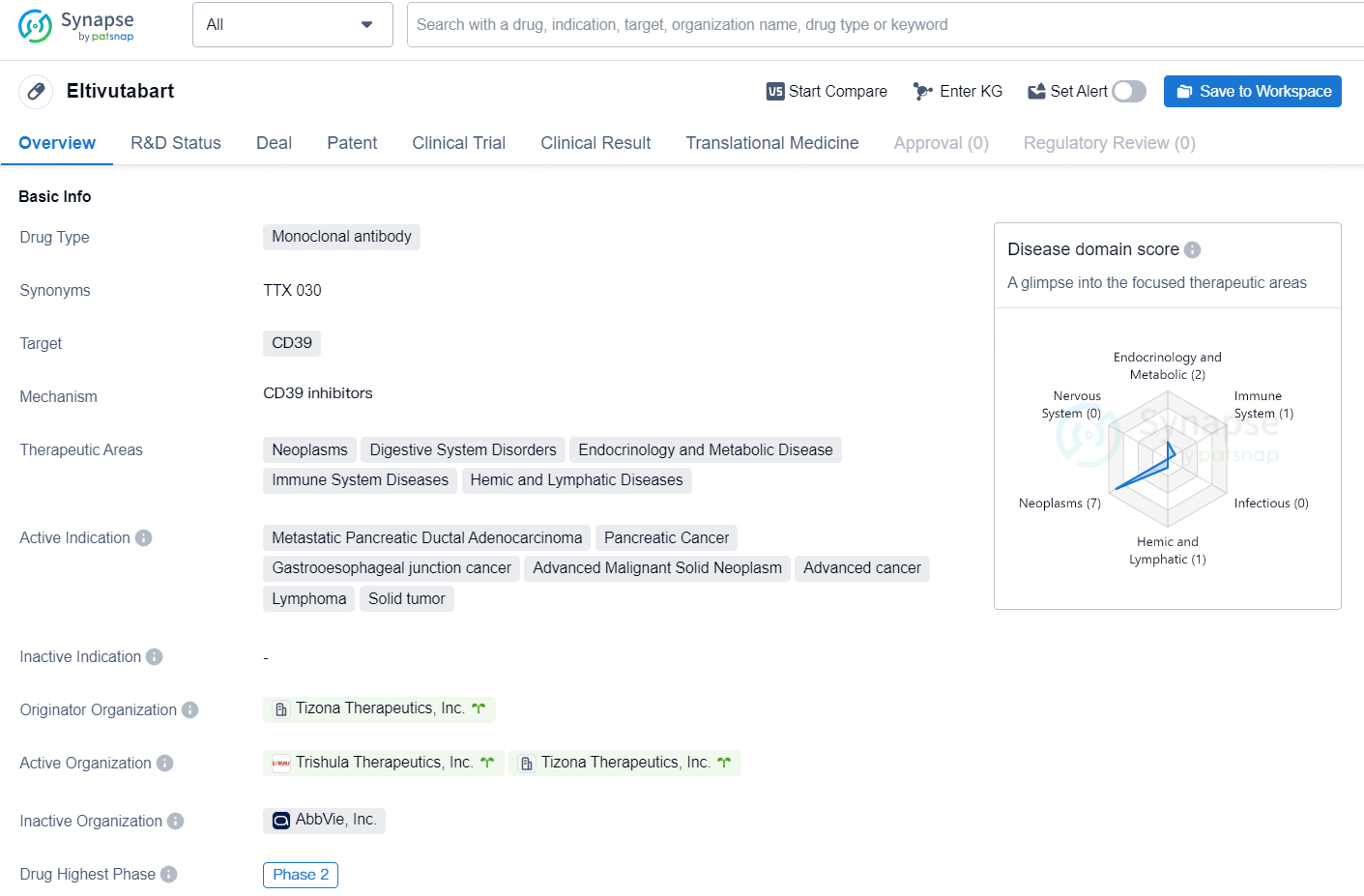
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The Phase 1 study outcomes revealed a notable median overall survival for individuals with metastatic pancreatic cancer treated with combinations involving TTX-030, especially in those exhibiting high tumor HLA-DQ biomarker levels," expressed Anil Singhal, CEO of Trishula Therapeutics. "These insights have driven the launch of our global, randomized Phase 2 ELTIVATE trial, with findings anticipated by 2026."
In the Phase 1 trial, TTX-030 was tested in combination with gemcitabine/nab-paclitaxel, and optionally budigalimab (a trial-stage anti-PD-1 antibody), as first-line therapy for pancreatic adenocarcinoma. Among the efficacy-evaluable group (n=57), consisting of 92% first-line metastatic and 8% locally advanced unresectable patients, the objective response rate (ORR) stood at 30%, including 3 complete responses; the median progression-free survival (mPFS) was 7.5 months (95% CI 5.2, 9.4); and the median overall survival was 19.1 months (9.8, NR). Examination of pre-treatment tumor samples indicated a subgroup of patients with high HLA-DQ expression linked to better clinical results with TTX-030 combinations. For the 28 HLA-DQhigh patients, the ORR was 46%, mPFS was 9.6 months (95% CI 3.9, 11.8), and mOS was 21.9 months (9.8, NR).
Both therapeutic combinations were well-tolerated, with only five patients (8%) halting treatment due to adverse events (AEs). The most common AEs were those anticipated from standard chemotherapy regimens, without an increase in frequency or severity.
"Previous evaluations of immune checkpoint therapies have shown limited effectiveness in this patient group, necessitating new strategies. These results are very promising, especially for patients with high biomarker expression, and justify further exploration of TTX-030 for advanced pancreatic cancer," stated Zev Wainberg, MD, Professor of Medicine at UCLA.
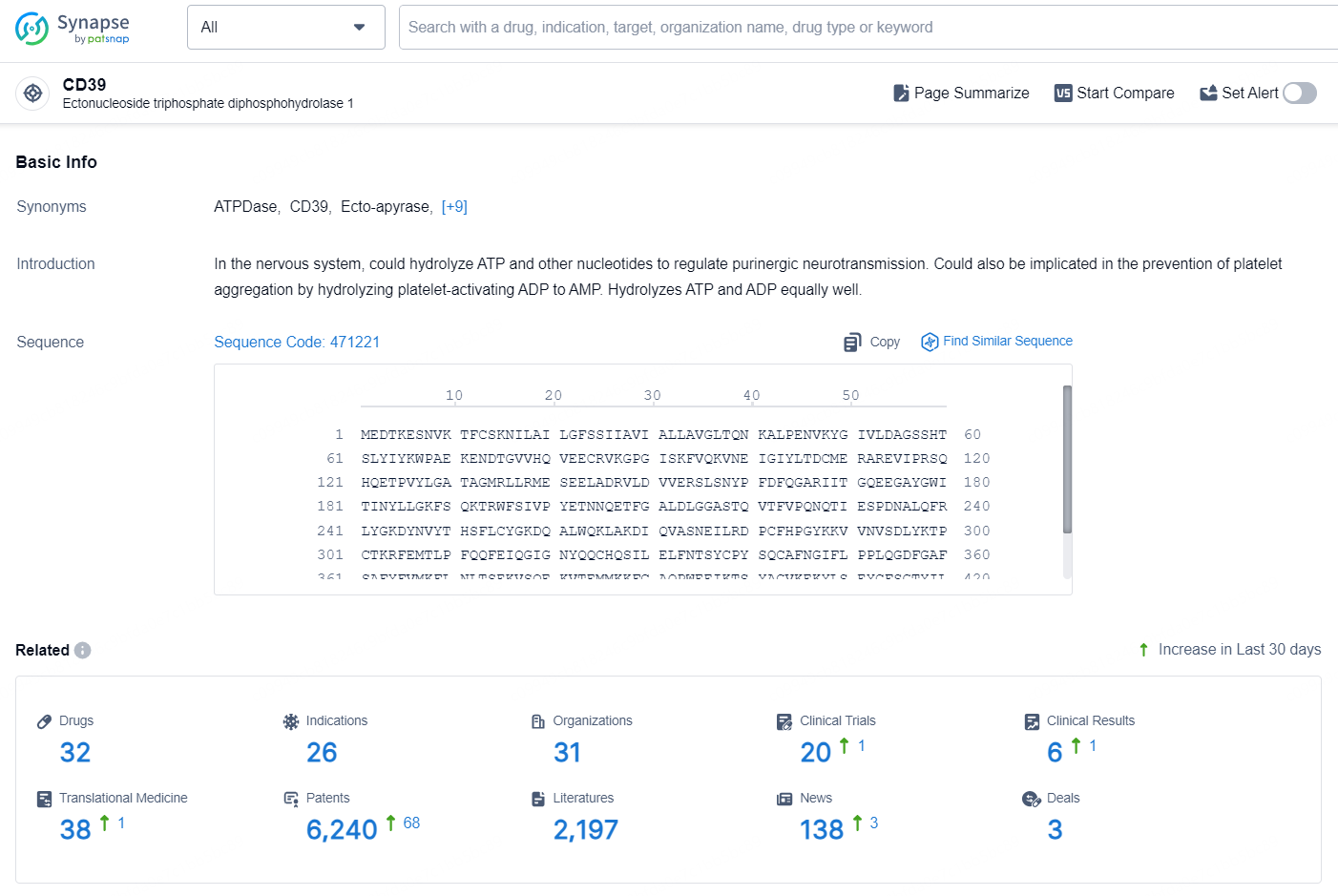
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 18, 2024, there are 32 investigational drugs for the CD39 targets, including 26 indications, 31 R&D institutions involved, with related clinical trials reaching 20, and as many as 6241 patents.
The drug Eltivutabart is a monoclonal antibody that targets CD39. It falls under the therapeutic areas of neoplasms, digestive system disorders, endocrinology and metabolic disease, immune system diseases, and hemic and lymphatic diseases. Eltivutabart is indicated for the treatment of metastatic pancreatic ductal adenocarcinoma, pancreatic cancer, gastroesophageal junction cancer, advanced malignant solid neoplasm, advanced cancer, lymphoma, and solid tumor.