Kelun-Biotech's Sac-TMT Approved in China for Advanced TNBC Treatment
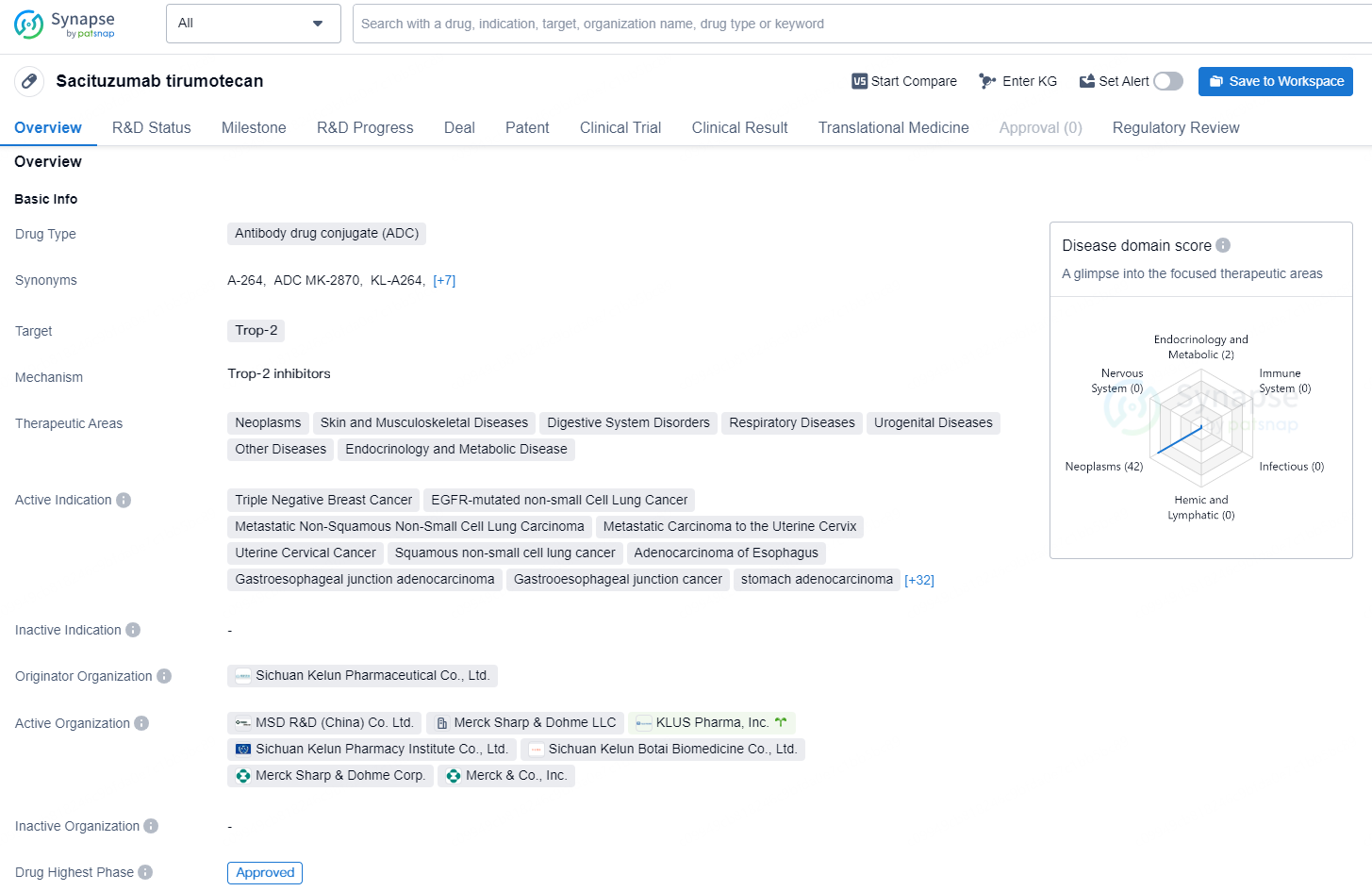
Sichuan Kelun-Biotech Biopharmaceutical Co., Ltd. has announced that it has received marketing approval in China from the National Medical Products Administration (NMPA) for sacituzumab tirumotecan (sac-TMT, previously known as SKB264/MK-2870), the first TROP2-directed antibody-drug conjugate (ADC) developed in the country. This approval is for adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) who have undergone at least two previous systemic treatments, with at least one targeting advanced or metastatic disease. This marks the first marketing approval in China for a domestically developed TROP2 ADC and also the first fully approved ADC produced in the country.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The authorization is grounded in favorable findings from a randomized, controlled, phase 3 trial, known as the OptiTROP-Breast01 study, involving adult individuals with unresectable locally advanced or metastatic TNBC who have undergone at least two prior systemic treatments (including one for the advanced or metastatic setting). Sac-TMT showed statistically significant and clinically relevant enhancements in both progression-free survival (PFS) and overall survival (OS) in comparison to chemotherapy. These findings were shared at a dedicated clinical science symposium focusing on next-generation ADCs during the 2024 American Society of Clinical Oncology (ASCO) Annual Meeting in May 2024.
Previously, the NMPA has accepted two supplemental new drug applications (sNDA) for sac-TMT monotherapy aimed at treating patients with locally advanced or metastatic EGFR-mutant non-small cell lung cancer (NSCLC) following disease progression on EGFR-TKI therapy alone, or after both EGFR-TKI and platinum-based chemotherapy.
Dr. Micheal Ge, CEO of Kelun-Biotech, expressed, “I am delighted to announce the crucial milestone of the successful approval and introduction of sacituzumab tirumotecan in China, which marks a significant accomplishment stemming from Kelun-Biotech’s extensive efforts in innovative development. As the company's first proprietary TROP2 ADC innovative therapy, the launch of sacituzumab tirumotecan paves the way for a new treatment approach for patients with 2L+ advanced TNBC. We anticipate that its remarkable clinical efficacy and safety profile will greatly benefit patients and enhance their quality of life. In the future, we will further investigate the clinical potential of sacituzumab tirumotecan across additional indications, fully leveraging its market potential to meet the clinical requirements of patients across the nation.”
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
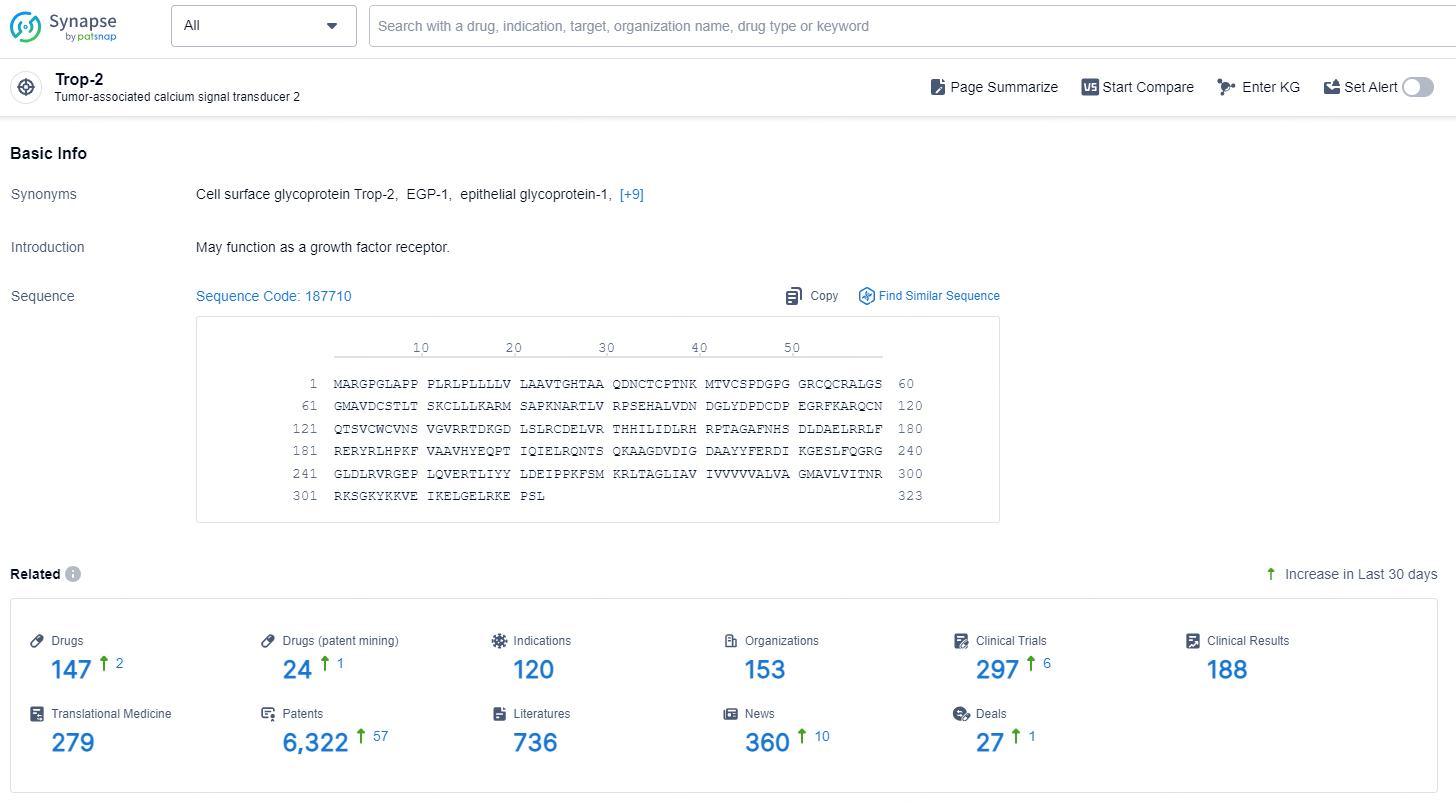
According to the data provided by the Synapse Chemical, As of December 2, 2024, there are 288 investigational drugs for the Trop-2 target, including 53 indications, 139 R&D institutions involved, with related clinical trials reaching 149, and as many as 21485 patents.
Sacituzumab tirumotecan is an antibody drug conjugate (ADC) primarily targeting Trop-2 and is used for the treatment of various neoplastic, skin and musculoskeletal diseases, digestive system disorders, respiratory diseases, urogenital diseases, other diseases, as well as endocrinology and metabolic diseases.