Weekly Insulin Efsitora Alfa Reduces A1C and Matches Daily Insulin Safety
Eli Lilly and Company revealed encouraging topline findings from the QWINT-2 and QWINT-4 phase 3 clinical studies, which assessed the efficacy of once-weekly insulin efsitora alfa (efsitora) in adults with type 2 diabetes initiating insulin therapy and those needing multiple daily injections of insulin. In the treat-to-target trials, efsitora demonstrated an A1C reduction that was non-inferior compared to the world's most widely used daily basal insulins.
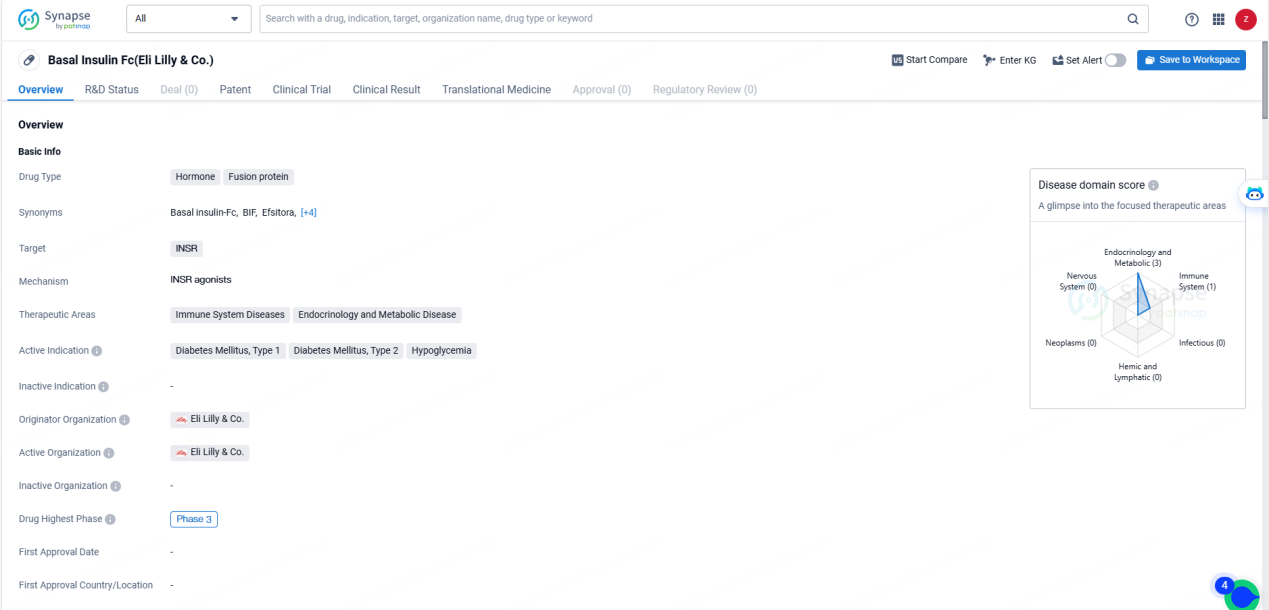
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"The findings from QWINT-2 and QWINT-4 mark a substantial achievement for the diabetes community, illustrating that efsitora, administered weekly, delivers blood sugar regulation comparable to daily basal insulins," stated Jeff Emmick, MD, Ph.D., senior vice president of product development at Lilly. "Efsitora offers a groundbreaking weekly insulin option that ensures A1C control, eases the burden of daily injections, and has the potential to improve adherence among diabetic patients."
QWINT-2 was an investigation into the effectiveness and safety of weekly efsitora versus daily insulin degludec over 52 weeks. The study involved insulin-naïve adults with type 2 diabetes who were randomly assigned to receive either weekly efsitora or daily insulin degludec. The trial also explored efficacy in patients both using and not using GLP-1 receptor agonists.
QWINT-4 assessed the effectiveness and safety of efsitora compared to insulin glargine over 26 weeks in adults with type 2 diabetes, who had prior treatment with basal insulin and at least two daily mealtime insulin injections. Participants were randomized to receive either weekly efsitora or daily insulin glargine, both combined with insulin lispro.
In both QWINT-2 and QWINT-4, efsitora demonstrated safety and tolerability with estimated combined rates of severe or clinically significant hypoglycemic events per patient-year of exposure being 0.58 for efsitora compared to 0.45 for insulin degludec, and 6.6 for efsitora compared to 5.9 for insulin glargine.
Detailed outcomes from QWINT-2 will be unveiled at the European Association for the Study of Diabetes Annual Meeting in 2024. Initial results from QWINT-1, QWINT-3, and QWINT-5 are expected later this year.
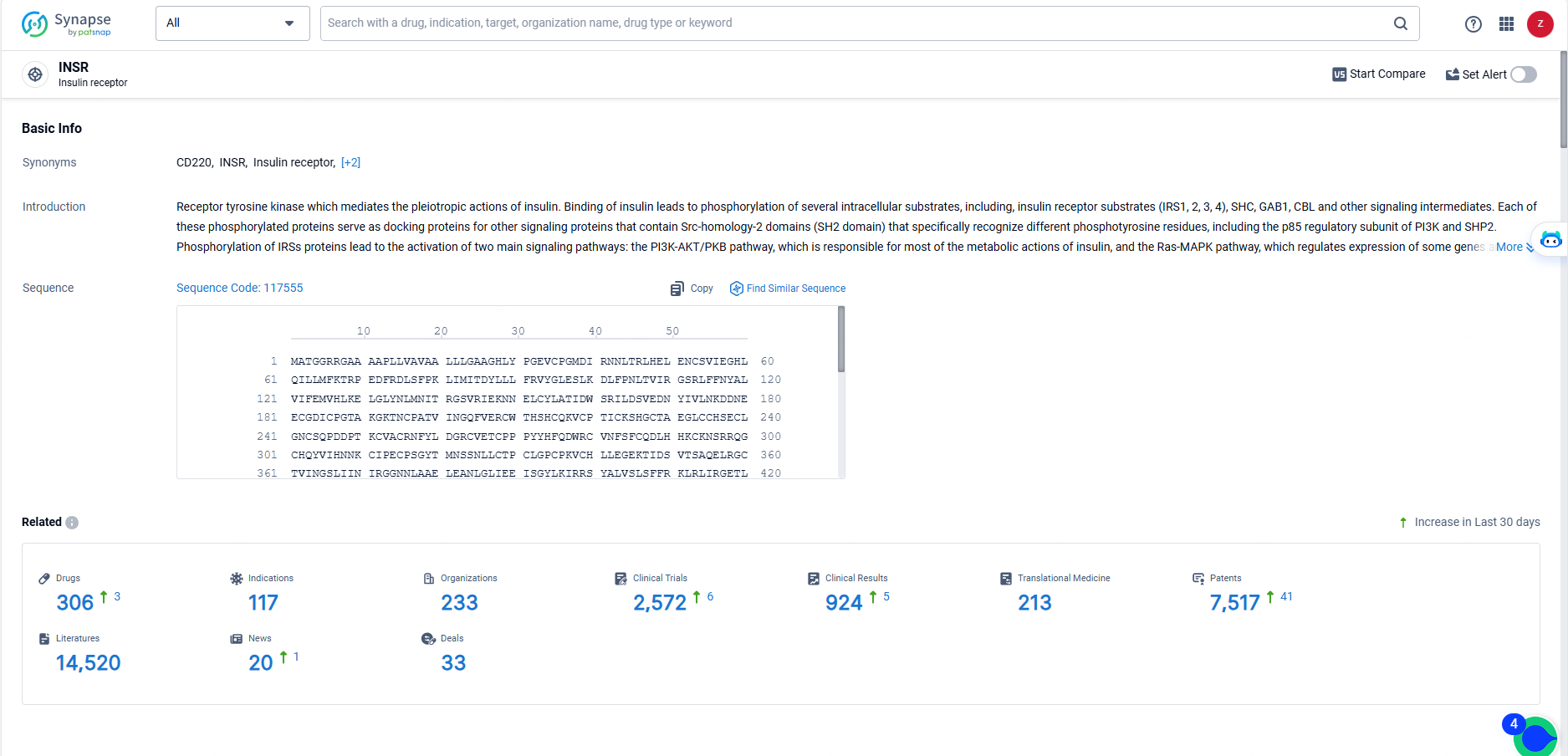
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 306 investigational drugs for the INSR target, including 117 indications, 233 R&D institutions involved, with related clinical trials reaching 2572, and as many as 7517 patents.
Basal Insulin Fc targets the insulin receptor (INSR) and is indicated for the treatment of Diabetes Mellitus, Type 1 and Type 2, as well as hypoglycemia. The therapeutic areas of focus for this drug include Immune System Diseases, Endocrinology, and Metabolic Disease.