A Comprehensive Review of Stiripentol's R&D Innovations and Drug Target Mechanism
Stiripentol's R&D Progress
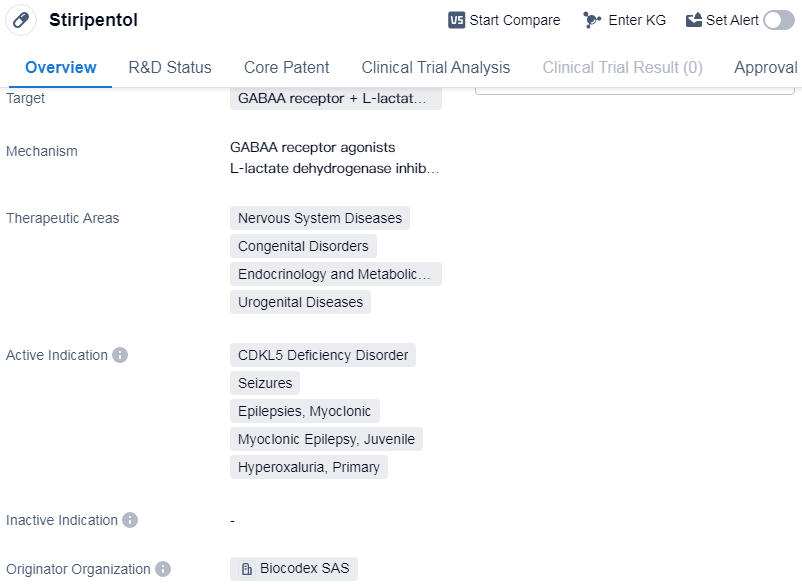
Stiripentol is a small molecule drug that targets the GABAA receptor and L-lactate dehydrogenase. It is primarily used in the treatment of various nervous system diseases, congenital disorders, endocrinology and metabolic diseases, and urogenital diseases. The drug has been approved for the treatment of CDKL5 deficiency disorder, seizures, epilepsies (specifically myoclonic and juvenile myoclonic epilepsy), and primary hyperoxaluria.
Stiripentol was developed by Biocodex SAS and received its first approval in the European Union in January 2007. The drug has reached the highest phase of development, which is the approved stage. This indicates that it has successfully completed clinical trials and has been deemed safe and effective for use in patients.
In terms of regulatory status, Stiripentol has undergone priority review and has been designated as an orphan drug. Priority review is a regulatory pathway that expedites the review process for drugs that address unmet medical needs. Orphan drug designation is granted to drugs that are intended to treat rare diseases or conditions, providing incentives to encourage their development.
Stiripentol's approval in the European Union in 2007 marked an important milestone in the treatment of CDKL5 deficiency disorder, seizures, and certain types of epilepsy. These conditions can have a significant impact on patients' quality of life, and the approval of Stiripentol provides healthcare professionals with an additional treatment option.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Stiripentol: GABAA receptor agonists and L-lactate dehydrogenase inhibitors
GABAA receptor agonists are a type of drugs that bind to and activate the GABAA receptors in the brain. GABAA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), which is the main inhibitory neurotransmitter in the central nervous system. When GABAA receptors are activated by agonists, such as certain drugs, they enhance the inhibitory actions of GABA, leading to a decrease in neuronal excitability and a calming effect on the brain. GABAA receptor agonists are used in the treatment of various conditions, including anxiety disorders, insomnia, and epilepsy.
L-lactate dehydrogenase inhibitors are substances that inhibit the activity of the enzyme L-lactate dehydrogenase. L-lactate dehydrogenase is an enzyme involved in the conversion of lactate to pyruvate during cellular metabolism. By inhibiting the activity of this enzyme, L-lactate dehydrogenase inhibitors can disrupt the normal metabolic processes that rely on lactate, potentially affecting energy production and other cellular functions. These inhibitors have been studied for their potential therapeutic applications in various diseases, including cancer, where altered lactate metabolism is often observed.
Drug Target R&D Trends for Stiripentol
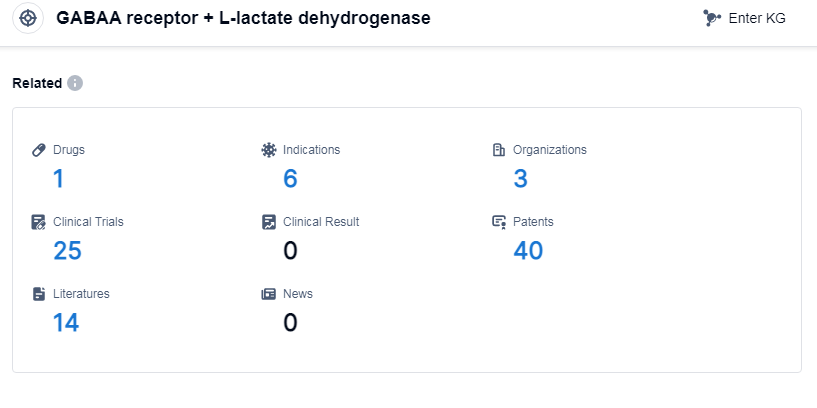
According to Patsnap Synapse, as of 8 Sep 2023, there are a total of 1 GABAA receptor and L-lactate dehydrogenase drugs worldwide, from 3 organizations, covering 6 indications, and conducting 25 clinical trials.
The analysis of the target GABAA receptor and L-lactate dehydrogenase reveals a competitive landscape with multiple companies actively involved in R&D. Biocodex SAS, Meiji Holdings Co., Ltd., and Jazz Pharmaceuticals Plc are among the companies with the highest phase drugs for this target. The approved indications include various forms of epilepsy, CDKL5 deficiency disorder, and primary hyperoxaluria. Small molecule drugs are the most prominent drug type in development. The countries/locations with the highest phase drugs include the European Union, United States, Liechtenstein, Taiwan Province, Australia, Iceland, Canada, Japan, Norway, Argentina, and France. This indicates a global interest and progress in the development of drugs targeting the GABAA receptor and L-lactate dehydrogenase. Further research and development in this area hold promise for the treatment of epilepsy and related disorders.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Stiripentol is a small molecule drug that targets the GABAA receptor and L-lactate dehydrogenase. It has been approved for the treatment of CDKL5 deficiency disorder, seizures, and certain types of epilepsy. Developed by Biocodex SAS, Stiripentol received its first approval in the European Union in 2007 and has undergone priority review as well as orphan drug designation. Its approval represents a significant advancement in the treatment of these conditions and provides healthcare professionals with an additional therapeutic option.