Recludix Pharma Presents Oral STAT3 Inhibitor Data in Th17 Skin Inflammation at SID Annual Meeting
Recludix Pharma, renowned for its pioneering work in identifying inhibitors for complex targets in the treatment of inflammatory diseases and cancer, revealed new findings today during a plenary oral session at the Society for Investigative Dermatology Annual Meeting. The presentation, entitled “Oral selective STAT3 inhibitors show distinct efficacy and safety profiles in preclinical models of Th17-mediated skin inflammation,” highlighted the promising results.
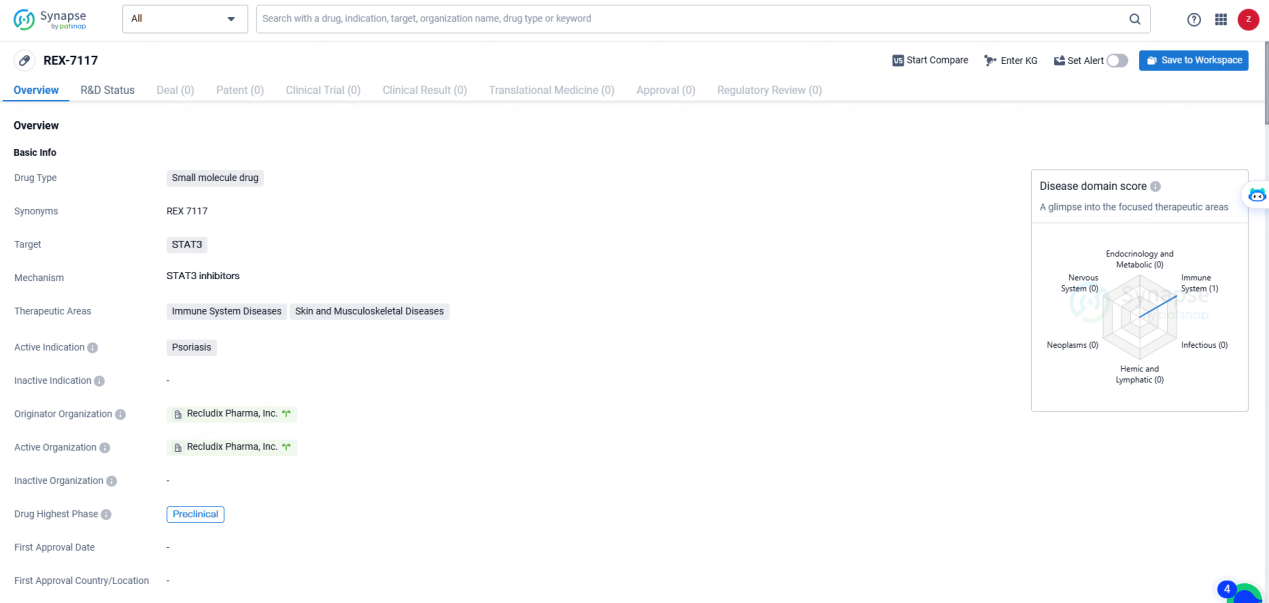
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Paul Smith, Ph.D., the senior vice president of biology at Recludix, has examined preclinical data related to the company’s potent, selective, and orally bioavailable small molecule STAT3 inhibitors, specifically REX-7117. The data revealed that REX-7117 provides profound, sustained, and targeted STAT3 inhibition, delivering efficacy comparable to IL-17-targeting biologics in in vivo models of plaque psoriasis. Unlike JAK1/2 and TYK2 inhibitors, REX-7117 does not compromise broader immune responses, such as interferon-dependent antiviral immunity or growth factor signaling vital for hematologic equilibrium.
“These compelling findings on our oral STAT3 inhibitors suggest that deep STAT3 inhibition can drive strong efficacy akin to clinically validated biologics, with the added benefit of oral administration convenience. The high selectivity for the downstream STAT3 target may also mitigate some safety issues seen with JAK and TYK2 inhibitors,” remarked Ajay Nirula, M.D., Ph.D., executive vice president and head of research and development.
In a murine psoriasis model induced by IL-23, REX-7117 demonstrated efficacy comparable to an anti-IL-17A antibody surrogate treatment and surpassed an estimated clinically relevant dose of deucravacitinib.
IL-23 and IL-6 cytokines signal through STAT3 to promote the development and function of pathogenic T helper type-17 (Th17) cells, which are pro-inflammatory immune cells. Th17 cells are crucial in various inflammatory diseases, such as rheumatoid arthritis, psoriasis, inflammatory bowel disease, among others. By inhibiting STAT3, there is potential for efficacy beyond Th17 / IL-17-driven diseases, particularly in conditions where blocking the IL-6 pathway is clinically validated. Additionally, clinical trials targeting oncostatin M and IL-22, both dependent on STAT3, have shown efficacy in inflammatory diseases.
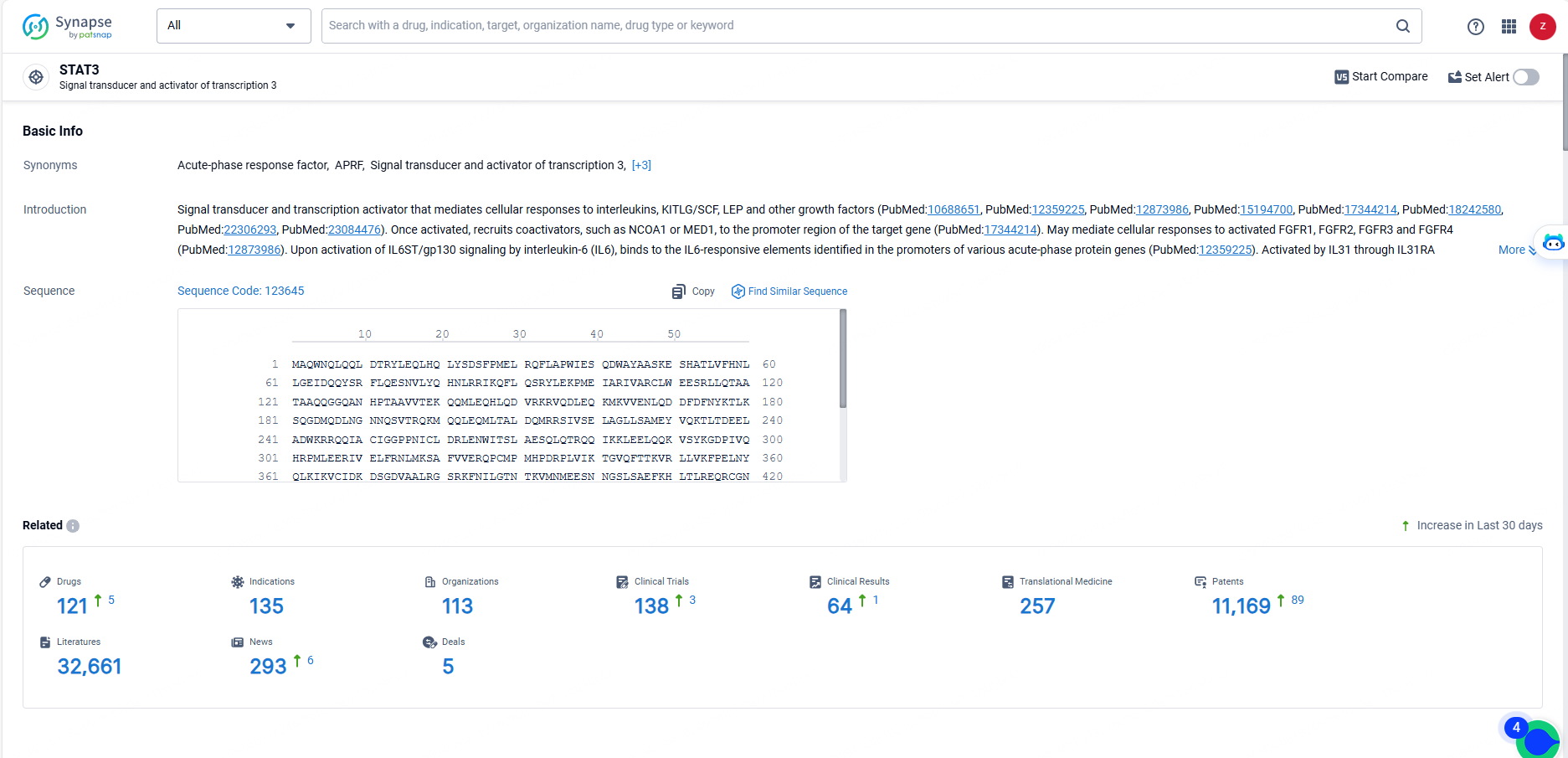
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of May 23, 2024, there are 121 investigational drugs for the STAT3 target, including 135 indications, 113 R&D institutions involved, with related clinical trials reaching 138, and as many as 11169 patents.
REX-7117 is a small molecule drug targeting STAT3 for the treatment of psoriasis and other immune-related diseases. With its current status in the preclinical phase, further research and development efforts will be needed to assess its potential as a therapeutic option for patients in need.