EMA Approves Italfarmaco Group's Application for Givinostat Use in Duchenne Muscular Dystrophy Treatment
Today, the Italfarmaco Group has publicly declared that they have submitted a Marketing Authorization Application for Givinostat. This drug may serve as a potential therapeutic solution for Duchenne Muscular Dystrophy. The submission was made to the European Medicine Agency, which has already initiated its regulatory review process concerning this application.
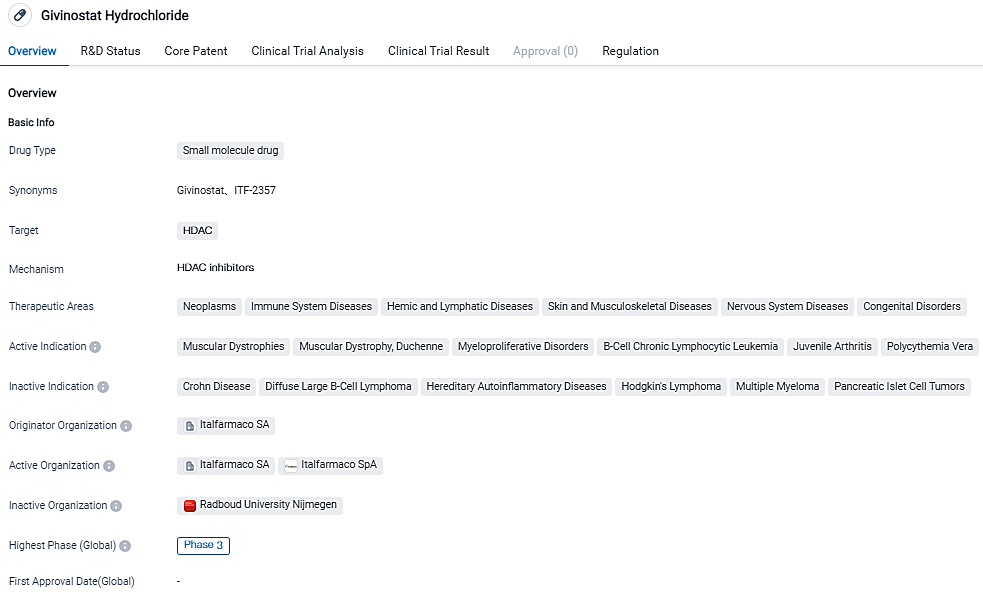
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The submission of MAA was constructed based on the safety and efficacy data derived from the Phase 3 EPIDYS clinical trial. This trial examined the effect of Givinostat, a histone deacetylase (HDAC) inhibitor, on DMD patients. Following the MAA application, the Italfarmaco Group filed an NDA for Givinostat with the FDA, which was granted a priority review, notified in June this year.
The critical Phase 3 clinical trial EPIDYS involved examining the safety and efficacy of Givinostat in a group of 179 DMD patients who were capable of walking and were six years and above. Givinostat was given orally for a period of 18 months, in combination with ongoing corticosteroid treatment. The study was successful, meeting its main goal of assessing functional progress through the average change from the initial reading in time taken to climb four stairs for the selected population. Released data from the safety and tolerability profile of Givinostat were in line with results from prior studies.
DMD patients experience detrimental changes in muscle tissue biological processes due to the lack of the dystrophin protein, leading to gradual muscle degeneration and eventually early death. Givinostat is capable of thwarting HDAC pathological overactivity. This consequently fixes the faulty sequence of intracellular signaling pathways that lead to muscle harm, reversing the disease pathology and decelerating muscle deterioration.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
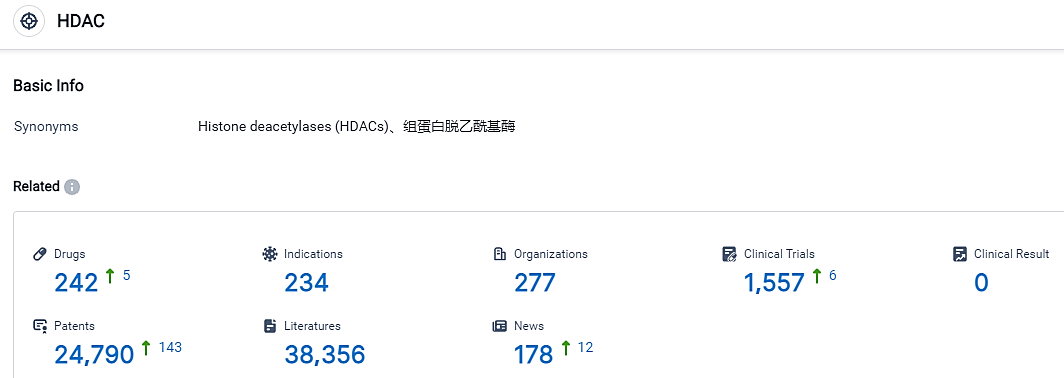
According to the data provided by the Synapse Database, As of September 7, 2023, there are 242 investigational drugs for the HDAC target, including 234 applicable indications, 277 R&D institutions involved, with related clinical trials reaching 1557, and as many as 24790 patents.
Givinostat, a drug under research, was identified as a result of the Italfarmaco Group's own R&D endeavors. It is currently undergoing evaluation for its potential safety and effectiveness in treating Duchenne- and Becker- Muscular Dystrophy. Observations have noted Givinostat’s potential to decelerate disease advancement by lessening inflammation, intramuscular fibrosis, and the necrosis of muscle tissue while also noticeably enhancing muscle mass. It also works to decrease fatty substitution.