Stoke Therapeutics provides data related to the ongoing clinical development of STK-001 for the treatment of Dravet syndrome
Stoke Therapeutics, Inc., a biotech firm committed to tackling serious diseases by increasing protein production through RNA-based therapies, reported today key points from presentations pertaining to the continued clinical research of STK-001 - a first-of-its-kind possible drug aimed at addressing the root cause of Dravet syndrome.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
 In a new development, the recently released analysis results from the current Phase 1/2a trials and the SWALLOWTAIL open-label expansion study are being unveiled in a science forum for the initial instance. Also, for the first time, a fresh pharmacokinetic analysis of 61 patients included in the STK-001 trials is being exhibited. It shows a link between increased STK-001 drug presence in the brain and significant decreases in seizure incidents over the course of time.
In a new development, the recently released analysis results from the current Phase 1/2a trials and the SWALLOWTAIL open-label expansion study are being unveiled in a science forum for the initial instance. Also, for the first time, a fresh pharmacokinetic analysis of 61 patients included in the STK-001 trials is being exhibited. It shows a link between increased STK-001 drug presence in the brain and significant decreases in seizure incidents over the course of time.
Dravet syndrome is a serious and gradually worsening genetic epilepsy signified by recurrent, prolonged and resistant seizures starting in the first year post birth. The ailment falls under the classification of a developmental and epileptic encephalopathy due to associated developmental lags and cognitive disorders.
The Chief Medical Officer of Stoke Therapeutics, Barry Ticho, M.D., Ph.D., said, “Our clinical data till now indicates that patients who received STK-001 treatment saw considerable improvements.” He further added, “The fresh data from our pharmacokinetic study gives us increased assurance in the effects observed with STK-001 and provides critical insights as we plan the pivotal scheme for STK-001. We’re excited to disclose more data during 2024's initial quarter and provide updates on our Phase 3 strategy to the Dravet community in the first half of 2024.”
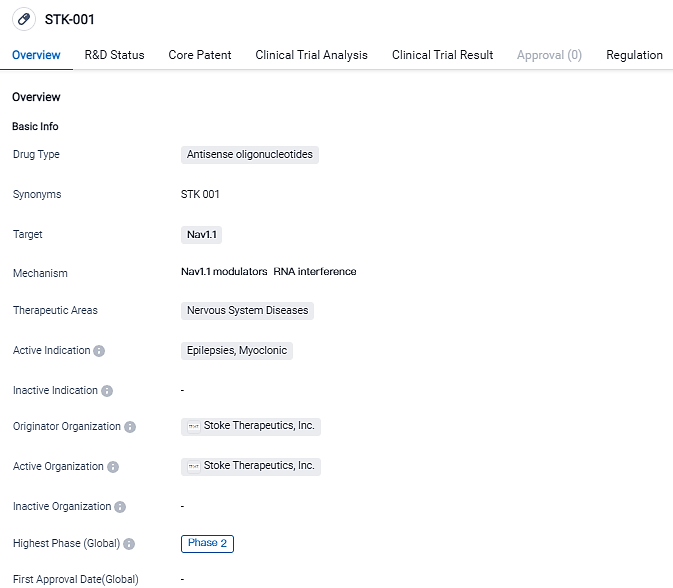
STK-001, a novel pharmaceutical under evaluation, is designed specifically for dealing with Dravet syndrome according to ongoing clinical studies. According to Stoke's prediction, STK-001 could be the initial therapeutic approach to tackle the genetic origins of Dravet syndrome. By employing the non-mutant copy of the SCN1A gene, STK-001 aims to elevate NaV1.1 protein expression leading to a restoration of normal NaV1.1 levels, thus decreasing both the frequency of seizures and substantial non-seizure comorbidities.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
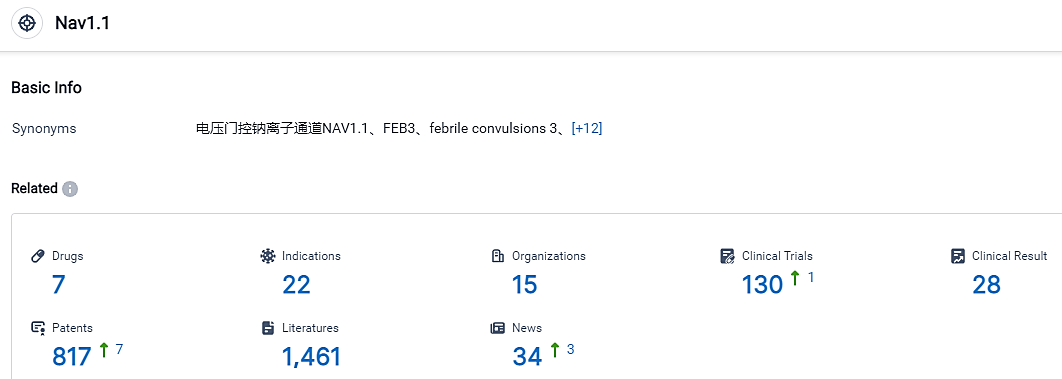
According to the data provided by the Synapse Database, As of September 7, 2023, there are 7 investigational drugs for the NaV1.1 target, including 22 applicable indications, 15 R&D institutions involved, with related clinical trials reaching 130, and as many as 817 patents.
Dravet syndrome is a dangerous and constantly developing hereditary epilepsy that undergoes regular, widespread, and persistent seizures from the first year of an individual's existence. This syndrome is considered a serious challenge for treatment and has a poor prognosis in the long run. Due to its association with developmental delay and cognitive impairment, this disease is designated as developmental and epileptic encephalopathy, and there is currently no officially recognized disease modification treatment method for Dravet syndrome patients.