Revolutionizing Gene Therapy: AI-Driven Advances in siRNA Drug Development
With advancements in gene-silencing technology, small interfering RNA (siRNA) is emerging as a promising therapeutic approach, increasingly approaching clinical application. However, designing efficient and highly specific siRNA sequences has remained a significant challenge for researchers. Leveraging the power of artificial intelligence (AI), scientists are exploring new methods to predict which siRNA sequences can most effectively silence target genes while minimizing off-target effects and immune responses. This AI-driven technological innovation not only enhances the targeting precision of siRNA but also paves new pathways for future gene therapies.
In siRNA drug development, accurate sequence prediction and optimization are crucial. AI and medical databases have demonstrated substantial potential in this area, enabling models to learn the characteristics of effective sequences from vast siRNA datasets and apply this knowledge to design new siRNA drugs. Through complex computational models, the combined use of AI and biomedical databases can assess the binding efficiency between siRNA and mRNA, predicting potential off-target risks and thus helping researchers design safer and more effective siRNA therapeutics.
AI-Assisted Sequence Design
Using machine learning models, AI can predict which siRNA sequences are most likely to effectively silence target genes, simultaneously reducing off-target effects and immune responses. AI can learn features of known effective sequences from existing siRNA databases and use these insights to design novel siRNA. This approach not only enhances siRNA targeting specificity but also reduces non-specific effects, thereby improving the therapeutic efficacy of siRNA. For instance, advanced AI design tools can analyze siRNA and mRNA sequences via sophisticated computational models, taking into account thermodynamic stability, sequence preference, and other biological factors to predict siRNA's inhibitory effects and potential off-target risks.
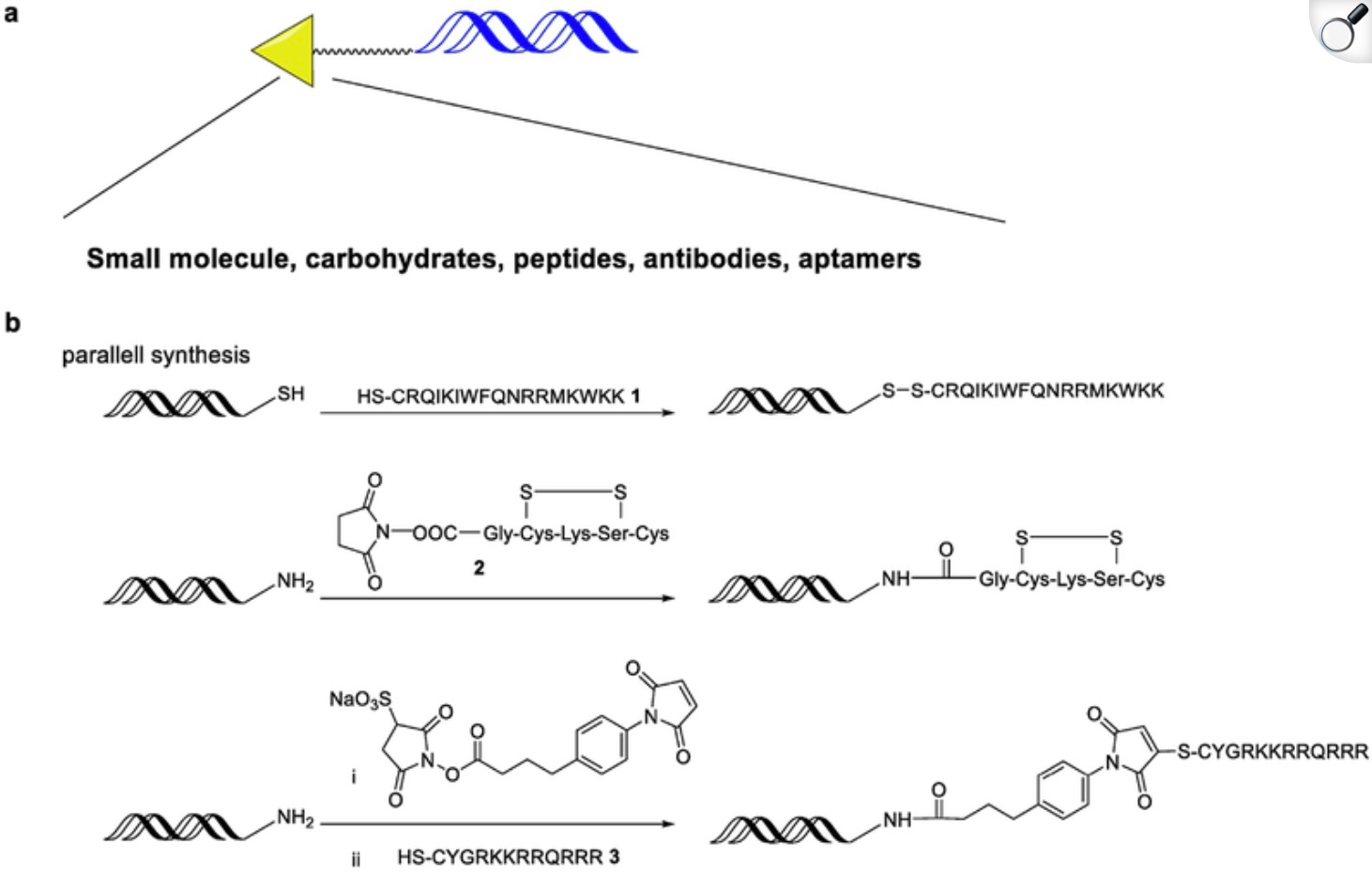
AI also aids in predicting potential off-target effects of siRNA sequences by analyzing their homology with non-target regions in the genome, thereby reducing possible adverse reactions. Researchers can use AI algorithms to examine the homology between siRNA sequences and off-target sites in the genome, identifying potential off-target sites in advance. This not only helps researchers identify risks early but also guides them in optimizing siRNA design for greater specificity and safety. Some predictive tools can generate RNA-DNA interaction models, developing effective off-target scoring methods to help researchers optimize siRNA sequences and reduce off-target risks.
AI Optimization of Delivery Systems
AI can also be applied to design chemical modification strategies to enhance siRNA stability in vivo and reduce its likelihood of degradation. By simulating the effects of various chemical modifications on siRNA stability, AI can help select optimal modification patterns. For example, AI can predict how chemical modifications affect the secondary structure of siRNA, thereby assessing its intracellular stability. AI models, trained on the relationship between known chemical modifications and siRNA stability, can provide customized chemical modification recommendations for new siRNA designs, ensuring that they remain active in complex in vivo environments.
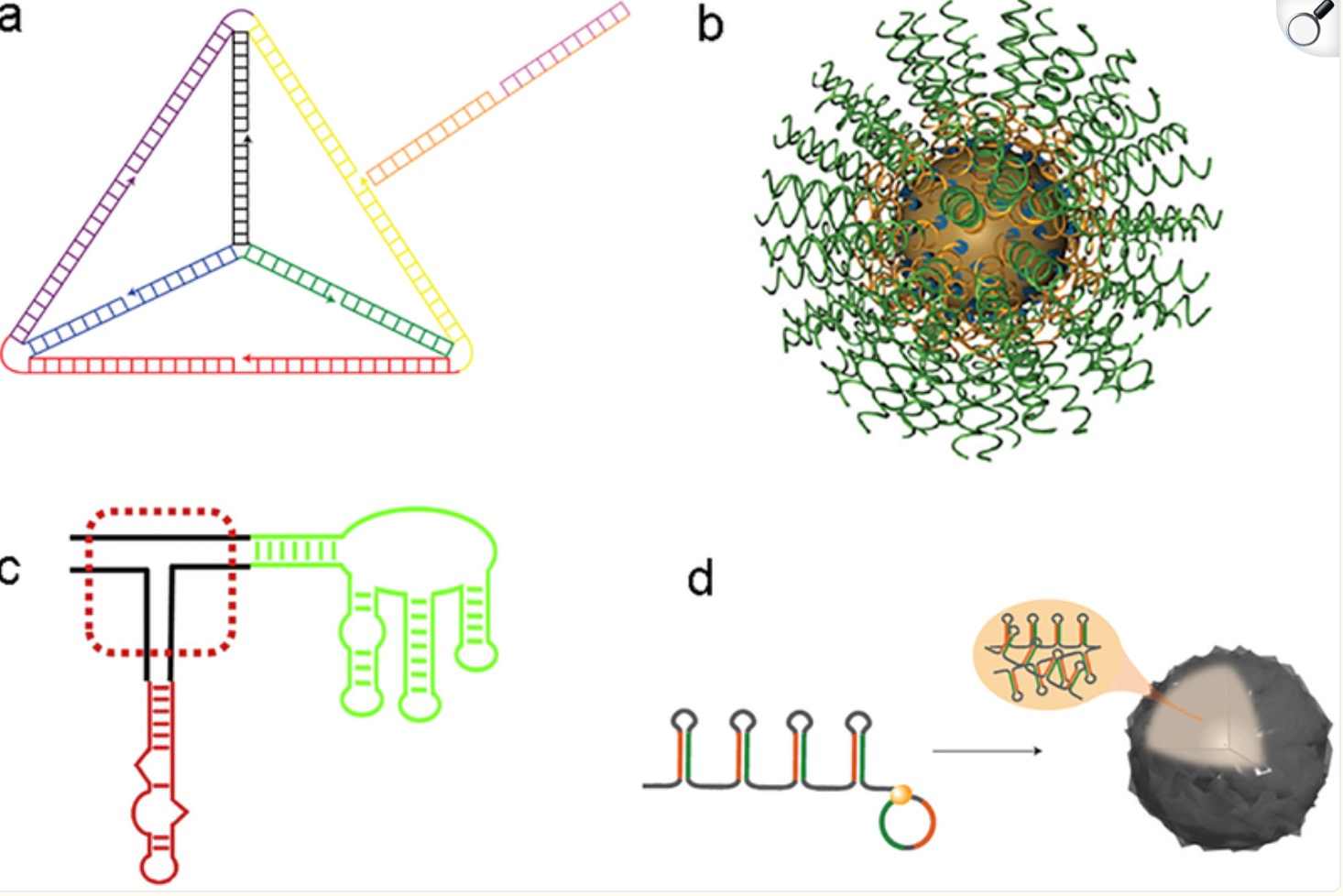
AI can also be utilized to optimize delivery carriers for siRNA, such as lipid nanoparticles (LNPs). By analyzing extensive data on delivery systems, AI assists in designing more efficient carriers that enhance siRNA targeting and bioavailability. For example, AI can analyze the composition ratios, structural characteristics of different LNPs, and their interactions with siRNA to predict which combinations will better protect siRNA from degradation and deliver it effectively to target cells. Moreover, AI can help screen delivery vehicles with specific tissue affinities, ensuring that siRNA reaches the affected area precisely and minimizing side effects from nonspecific binding.
Beyond AI, pharmaceutical databases play an essential role in supporting siRNA drug development, especially platforms like Patsnap, which provide extensive data resources and intelligent tools for siRNA R&D.
Patsnap Synapse in siRNA Drug Development
To explore the R&D landscape of siRNA drugs, begin by visiting the Patsnap Synapse website. After logging in, navigate to the search interface and enter keywords such as "siRNA" to view comprehensive insights into global siRNA drug development, including competitive landscapes, relevant patents, literature, and clinical trials.
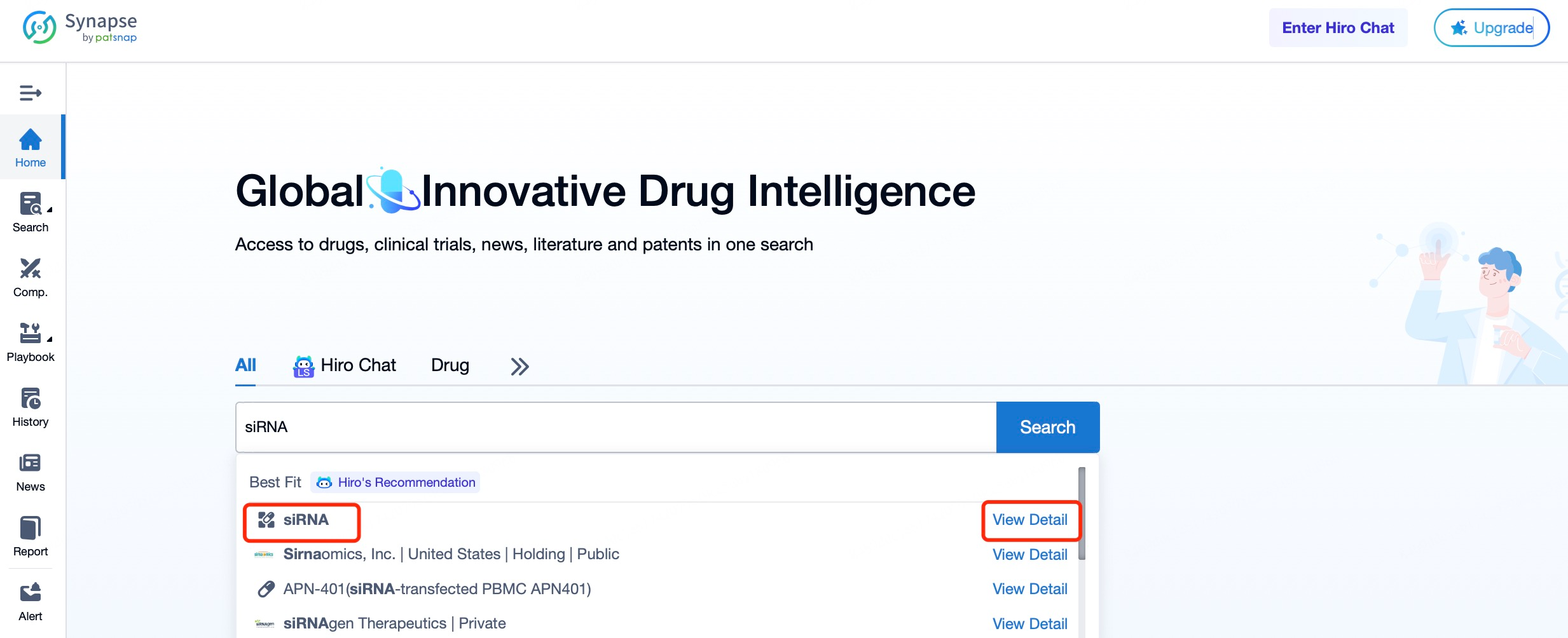
In the search results, users can easily access details on investigational and marketed siRNA drugs, including counts of investigational drugs, target numbers, and clinical data. The page also displays recent updates, such as the addition of 22 new siRNA drugs, 4 indications, 1 target, and 6 organizations in the past 30 days, along with the initiation of 3 new clinical trials and a published clinical report. These insights help users stay updated on the latest progress in siRNA therapeutics, reflecting the field’s growing interest and dynamic development.
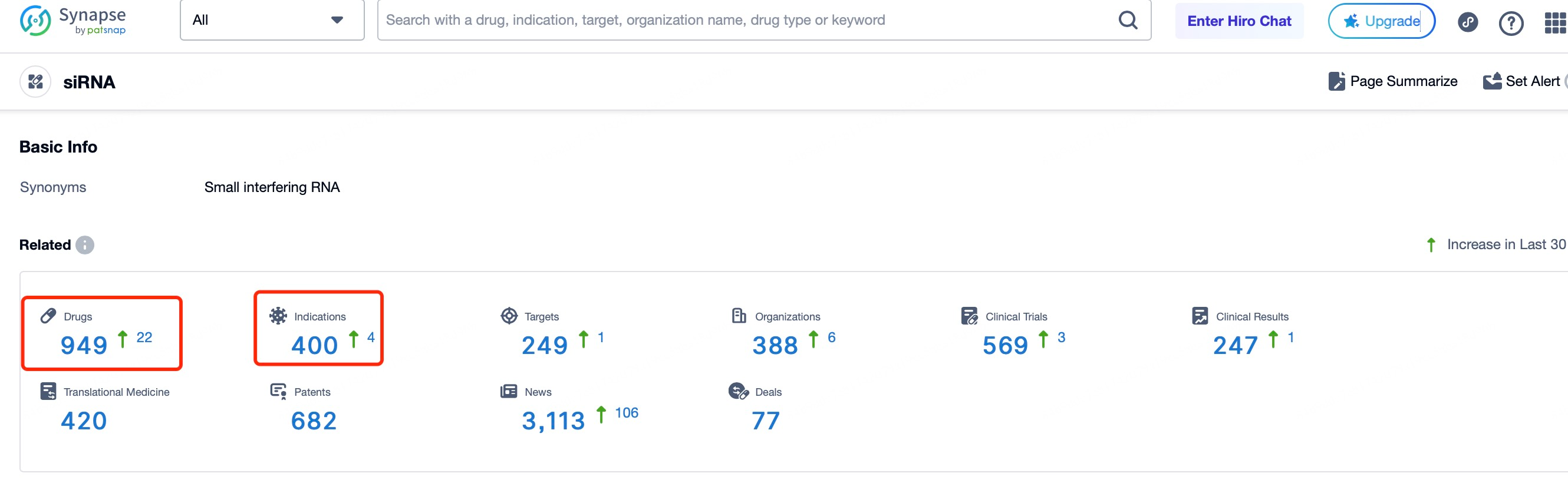
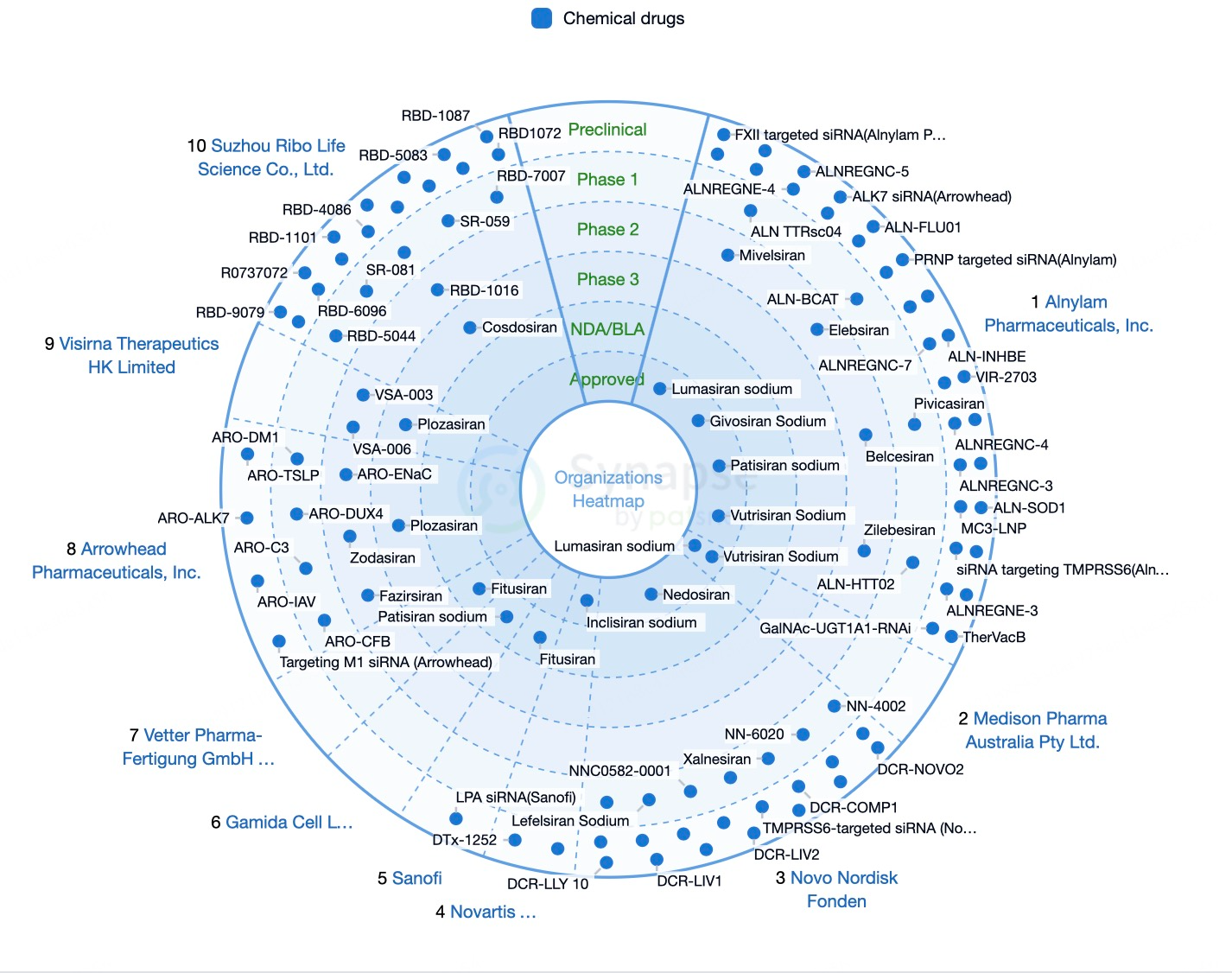
Patsnap Synapse's Bullseye diagram further visualizes the global status of marketed and investigational siRNA drugs. Marketed siRNA drugs are centered in the diagram, with different clinical stages radiating outward. Each layer’s color can represent the therapeutic area or the development company, offering researchers and investors a quick overview of which drugs have reached the market, those in key clinical phases, and the distribution across various development stages, aiding informed decision-making.
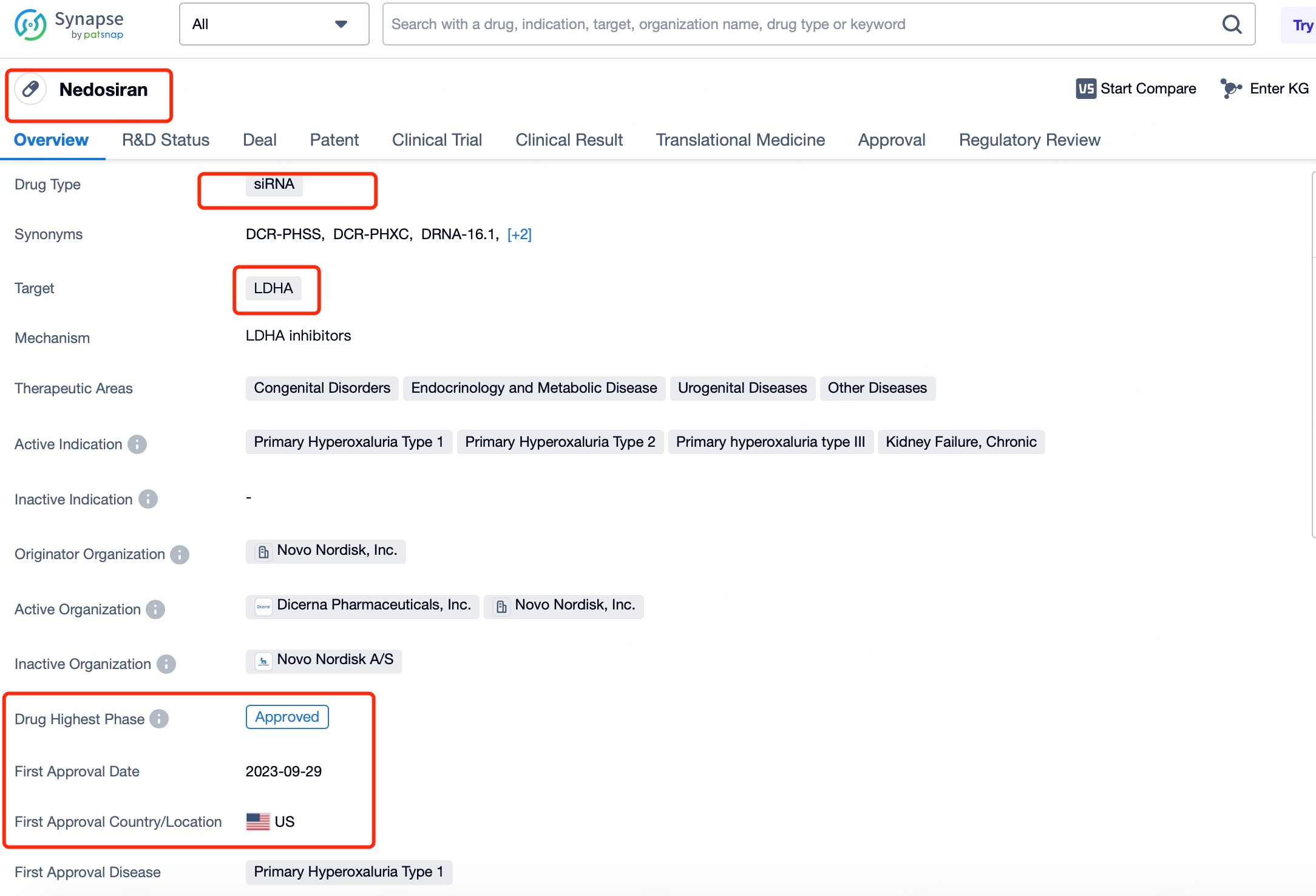
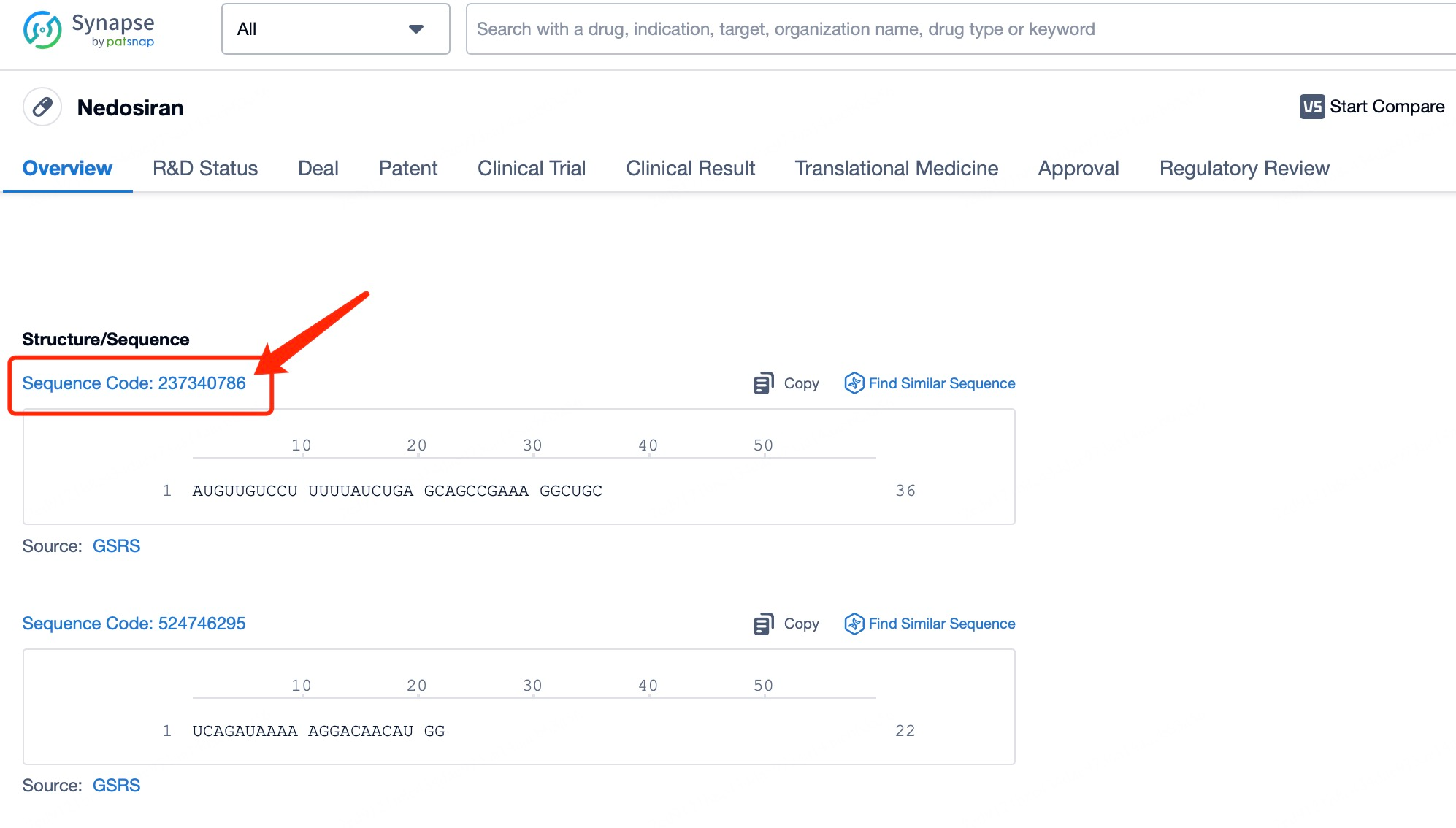
For specific siRNA drugs, users can search by name on Patsnap Synapse, such as by entering "Nedosiran" in the search bar. After clicking to view details, users can access comprehensive information on the drug’s background, development status, indications, and clinical trial progress. Advanced search options enable refined queries, like setting time frames, geographical regions, or document types, for more precise data retrieval. This approach not only provides an update on Nedosiran’s development but also offers graphical tools for data analysis, such as patent application trends and technology distribution charts, giving users a macro perspective on siRNA development trends.
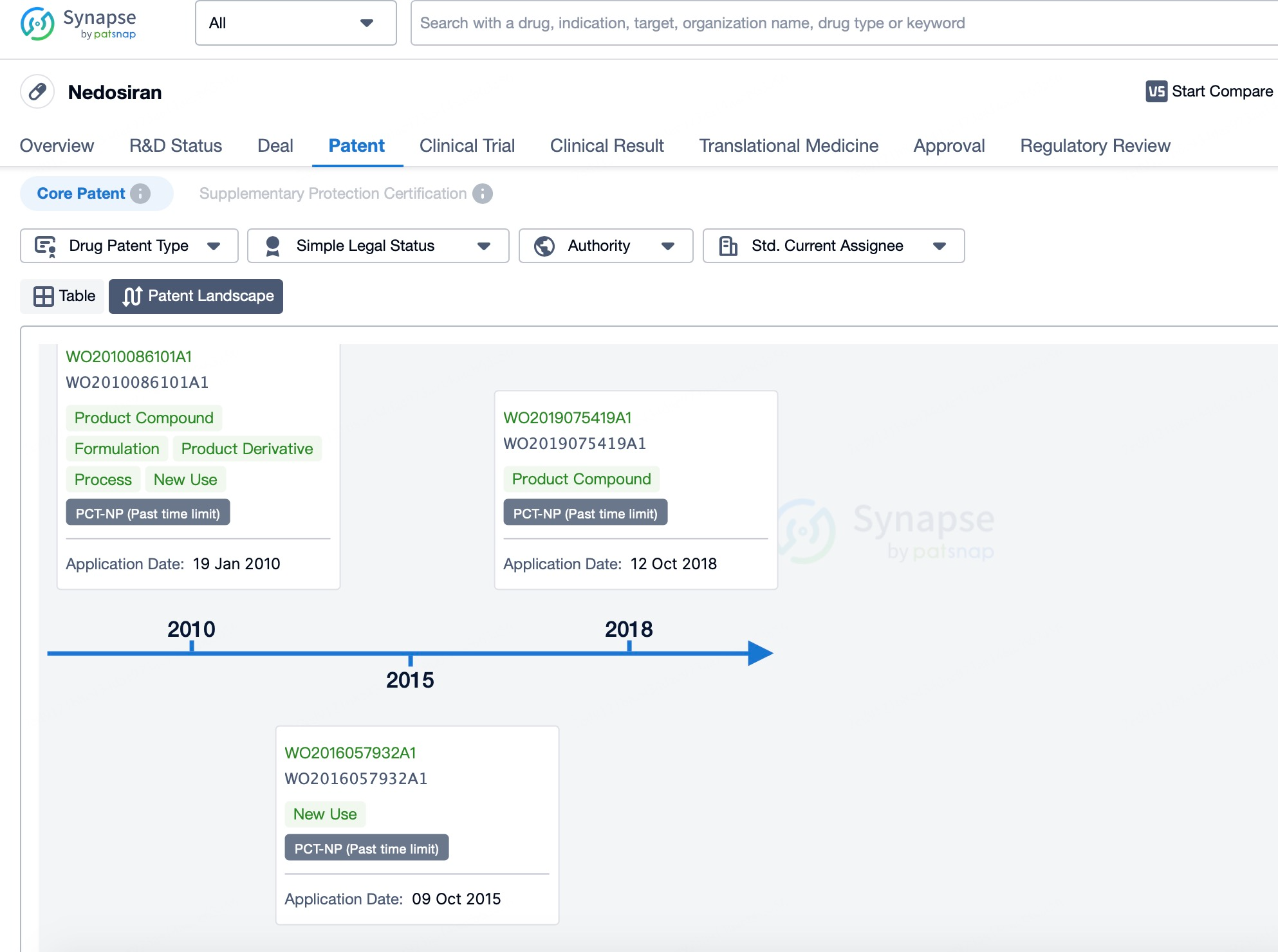
Additionally, Patsnap Synapse provides rich text-based resources along with timeline and graphical analysis tools. For instance, Patsnap Synapse can generate patent application timelines, trend charts, and technology distribution graphs, helping researchers understand the broader siRNA drug development landscape. These tools allow users to visualize siRNA technology trends globally or regionally, as well as different companies’ or research institutions’ investments and technological strengths. These graphical analyses offer users another way to interpret the data, equipping them with comprehensive information for better decision-making.
Once you’ve completed your search for basic siRNA drug information in Patsnap Synapse, you can seamlessly link to Patsnap Bio for additional details on the drug’s sequence information and chemical modifications. By clicking on the siRNA’s Sequence Code, you’ll be directed to Patsnap Bio, where you can access comprehensive sequence data and specific chemical modifications. This streamlined workflow not only simplifies information retrieval but also ensures data consistency and accuracy, providing strong support for your research.
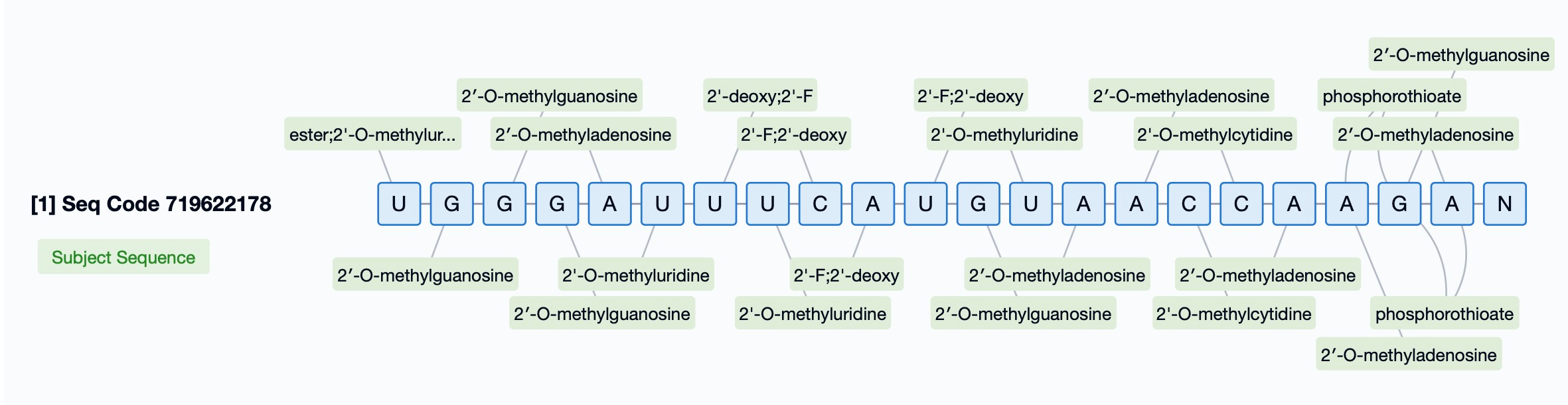
Patsnap Bio introduces a dedicated chemical modification search feature, enabling users to directly query specific types of chemical modifications. This feature is particularly beneficial for siRNA drugs, as it allows researchers to quickly identify any chemical modifications within sequences and understand their impact on drug performance. For example, users can apply filters to locate sequences with specific chemical groups, such as 2'-O-methyl modifications, or perform searches based on modification sites.
Large-scale biopharmaceutical model "Hiro-LS"
The large-scale biopharmaceutical model "Hiro-LS" plays a crucial role in siRNA drug R&D through its advanced AI-driven capabilities. By leveraging extensive literature and patent analysis, Hiro-LS helps researchers stay updated on the latest scientific advancements and technology trends. In target validation and sequence optimization, it provides precise data support and design recommendations to enhance drug efficacy and specificity. Additionally, "Hiro-LS" can suggest optimal chemical modifications to boost siRNA stability and delivery efficiency. The model also aids in preclinical and clinical data analysis, evaluates candidate drugs for safety and efficacy, and guides intellectual property strategy to ensure thorough protection of research outcomes. By fostering interdisciplinary collaboration, "Hiro-LS" accelerates the translation of basic research to clinical application, significantly improving R&D efficiency and innovation potential.
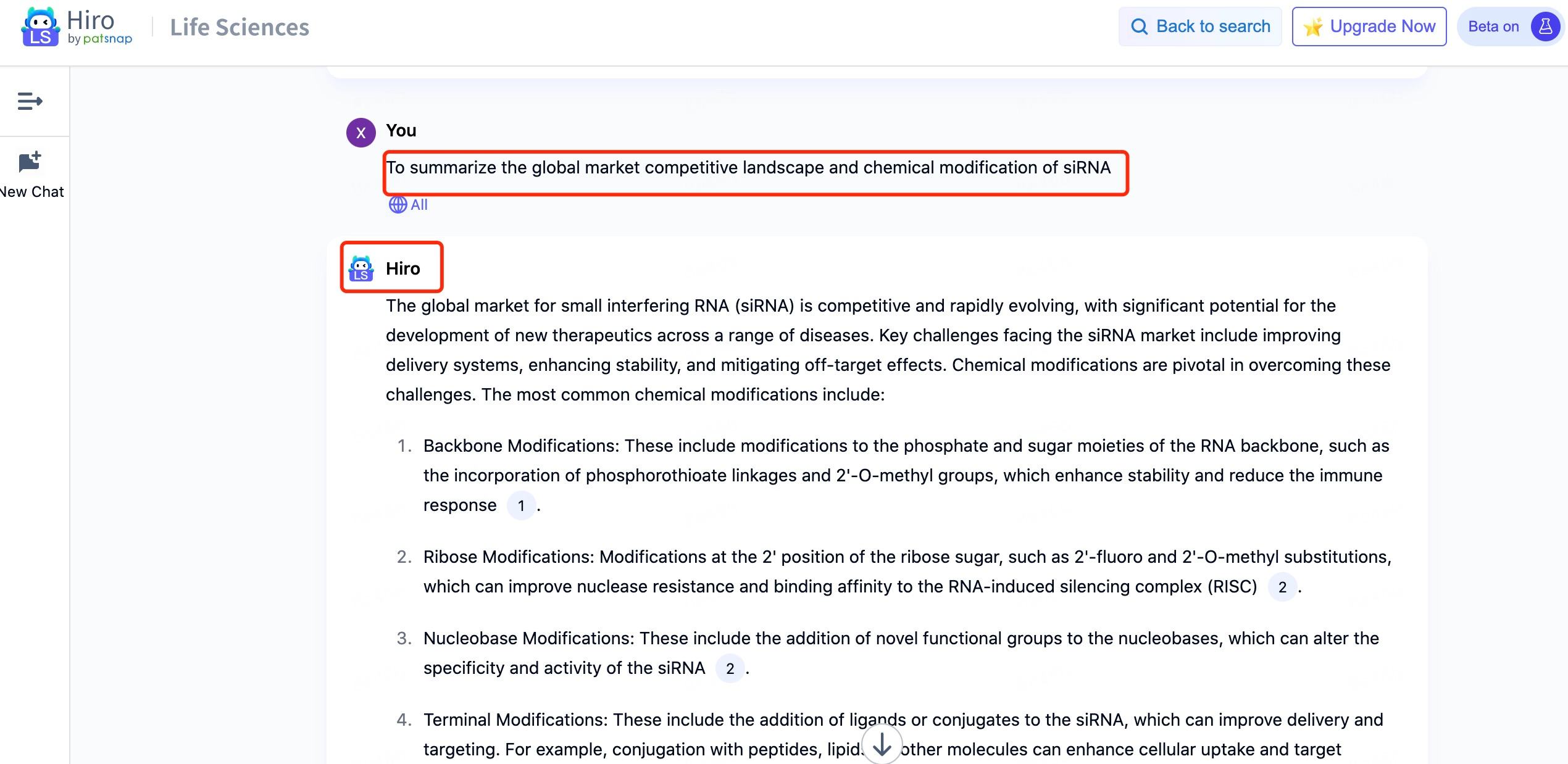
For an experience with the large-scale biopharmaceutical model Hiro-LS, please click here for a quick and free trial of its features!
Summary
In conclusion, AI plays a critical role in siRNA drug development, offering powerful tools and support across sequence design, off-target effect prediction, and chemical modification and delivery system optimization. These technologies not only enhance the efficiency of siRNA drug R&D but also greatly increase its therapeutic potential, laying a solid foundation for future precision medicine. By integrating AI and pharmaceutical databases into the siRNA drug development process, scientists achieve groundbreaking advancements in sequence design, off-target prediction, and chemical modification. This marks a significant leap in gene therapy, providing new hope for patients. As technology continues to evolve, we can expect more efficient and safe siRNA drugs to emerge, improving health outcomes for patients worldwide.
Reference:
- Dong, Y., D.J. Siegwart, and D.G. Anderson, Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev, 2019. 144: p. 133-147.