An analysis of Acimtamig's R&D progress and its clinical results presented at the 2023 ASH Annual Meeting
On 11 Dec 2023, the latest clinical trial of Innate Cell Engager (ICE®) AFM13 Combined with Preactivated and Expanded (P+E) Cord Blood (CB)-Derived Natural Killer (NK) Cells for Patients with Refractory CD30-Positive Lymphomas will be reported in 2023 ASH.
Acimtamig's R&D Progress
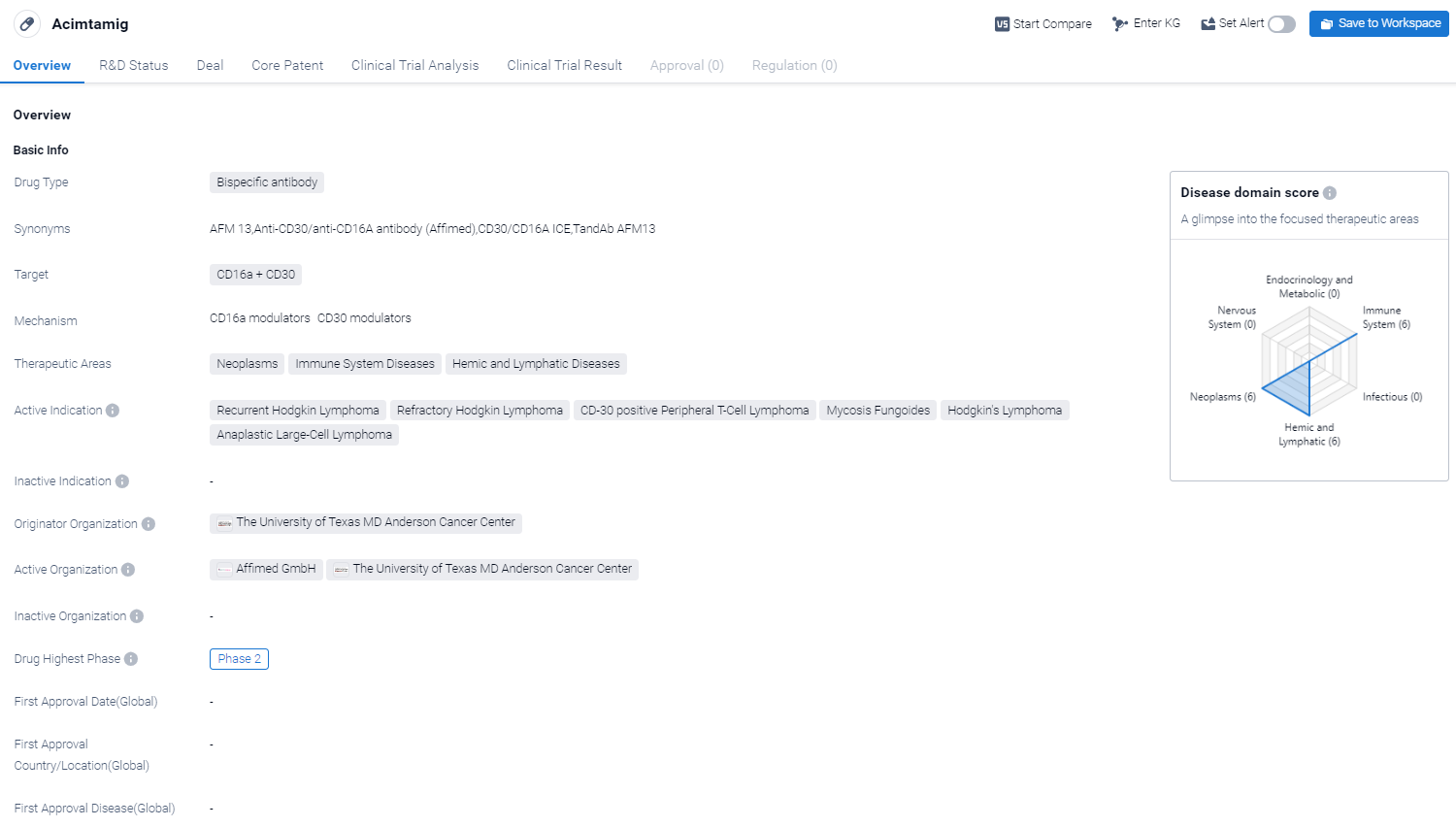
Acimtamig is a bispecific antibody drug that targets CD16a and CD30. It falls under the therapeutic areas of neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug is primarily indicated for recurrent Hodgkin lymphoma, refractory Hodgkin lymphoma, CD-30 positive peripheral T-cell lymphoma, mycosis fungoides, Hodgkin's lymphoma, and anaplastic large-cell lymphoma.
According to the Patsnap Synapse, Acimtamig has reached the highest phase of development, which is Phase 2 on a global scale. And the clinical trial areas for Acimtamig are primarily in the United States, France and Germany. The key indication is Mycosis Fungoides. 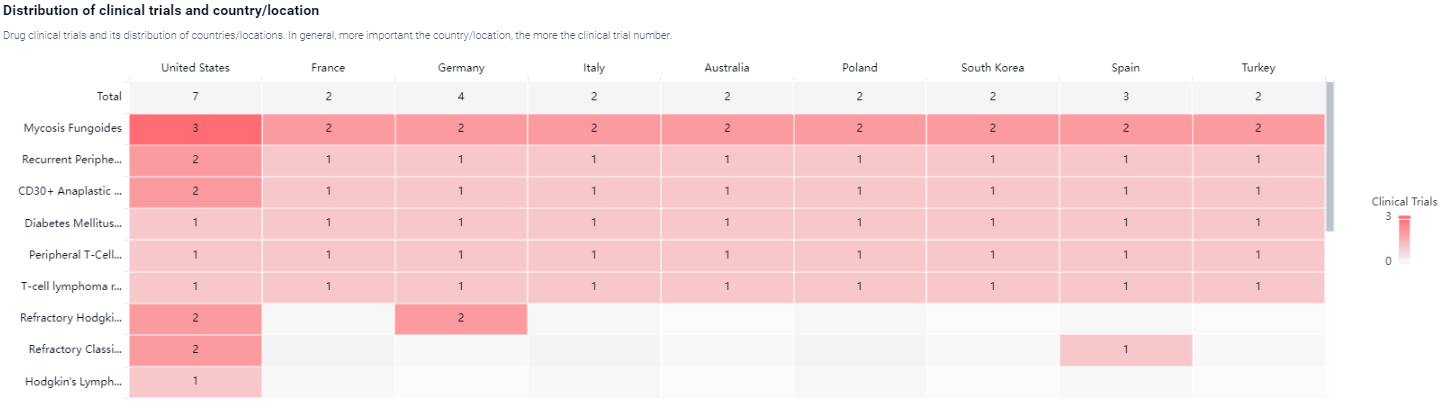
Detailed Clinical Result of Acimtamig
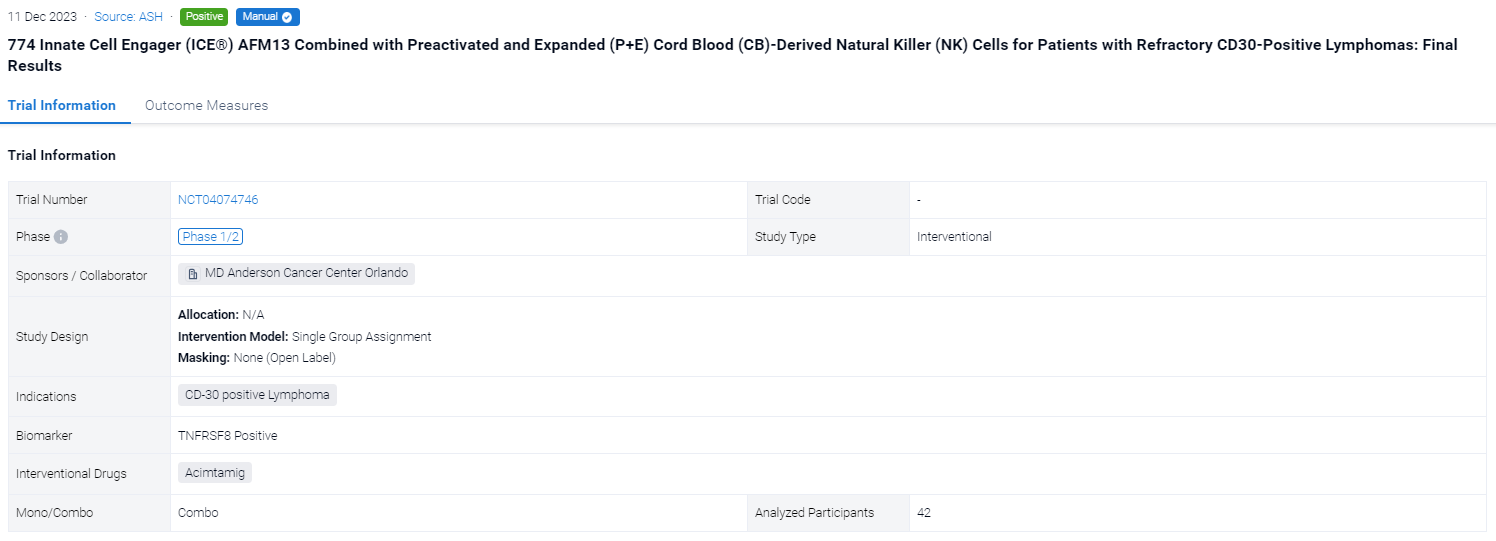
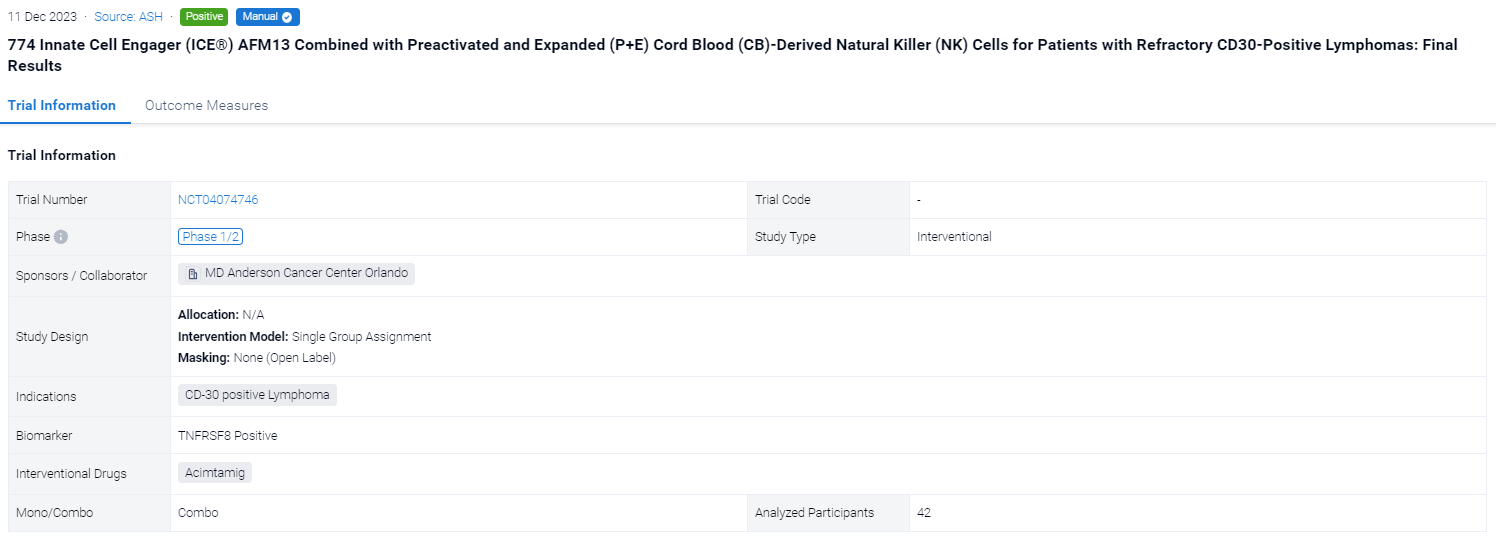
The single group assignment, open-labeled clinical trial (NCT04074746) was aimed to evaluated the safety and activity of P+E AFM13-precomplexed CB NK cells followed by systemic AFM13.
In this study, pts ages 15–75 with CD30+ lymphomas refractory to brentuximab vedotin were enrolled. The goals were to identify the recommended phase 2 dose (RP2D) of NK cells, estimate the overall (ORR) and complete response (CR), event-free (EFS) and overall survival (OS) rates, and study NK cell activation and persistence. Each treatment cycle consisted of fludarabine/cyclophosphamide (days −5 to −3) followed by infusion (day 0) of the AFM13-precomplexed CB NK cells, cultured for 14 days as described above, and three weekly IV infusions of AFM13 (200 mg, days 7, 14 and 21). Two cycles were planned for pts 1-19 and up to 4 cycles from patient 20 on. Response was evaluated on day 28 of each cycle. Enrollment proceeded at 3 dose levels (DL) of 106, 107 and 108 NK/Kg, respectively. Each patient received the same NK dose in all cycles.
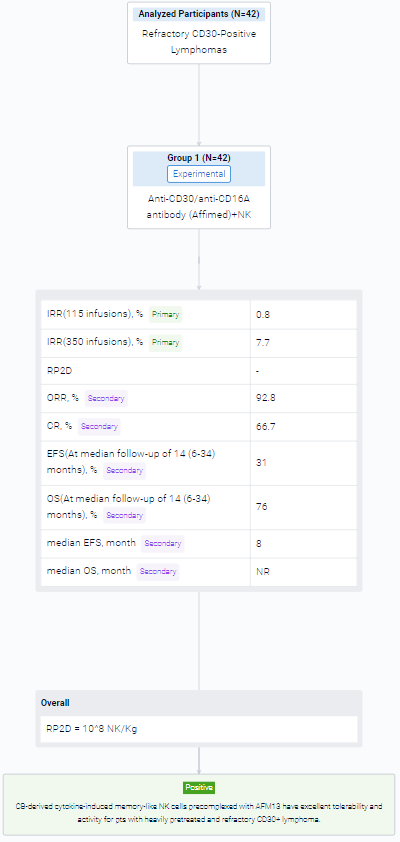
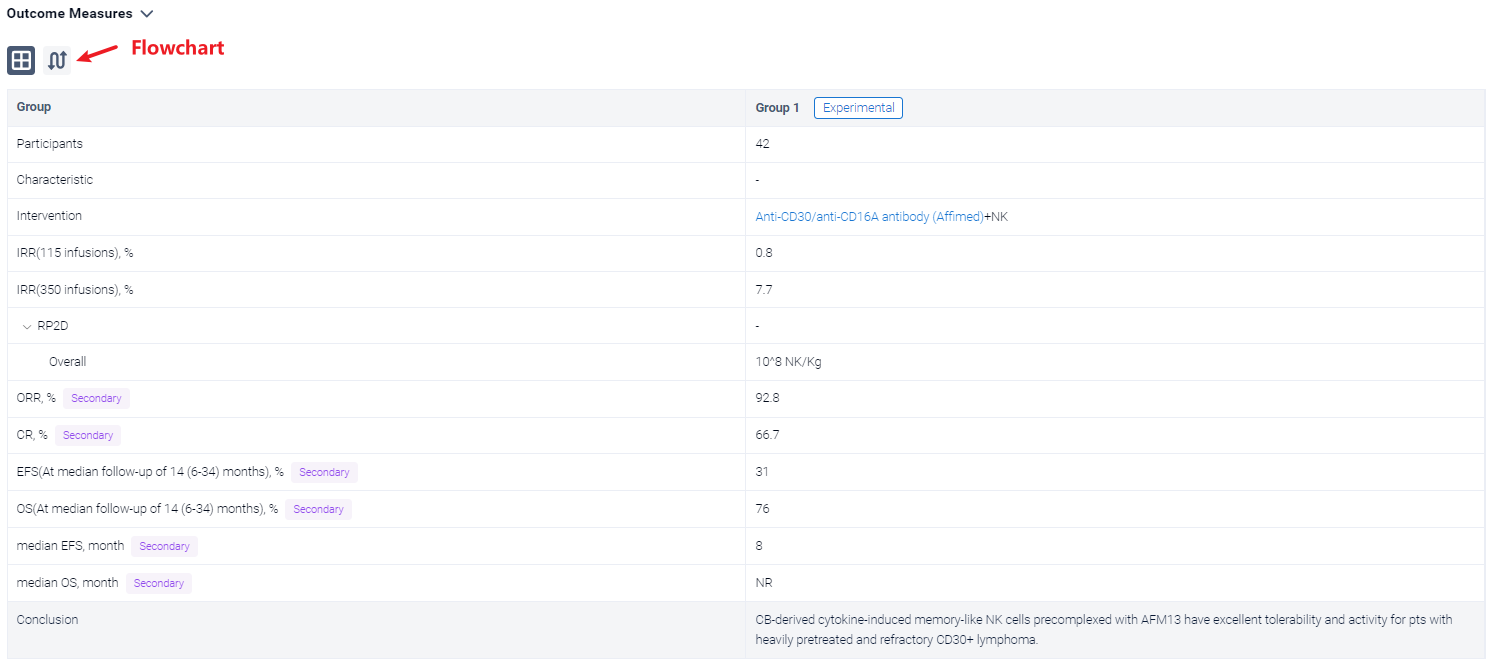
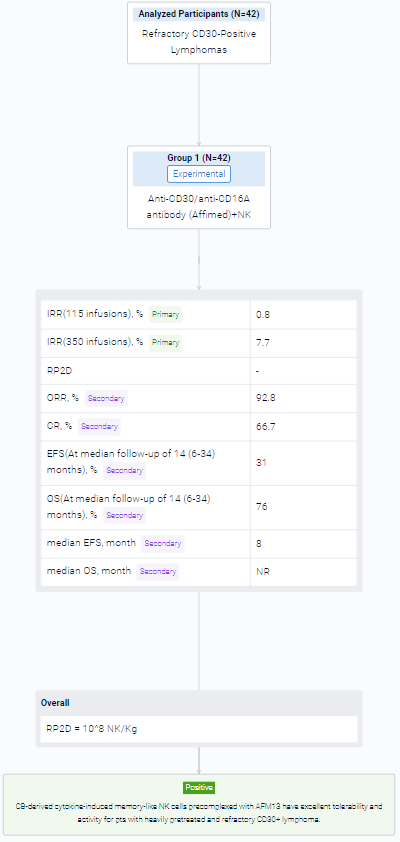
The result showed that between 09/20-12/22 we enrolled 42 pts (37 HL, 5 NHL), pretreated with a median 7 lines, at DL1 (N=3), DL2 (N=3) and DL3 (N=36), with 1 (N=2), 2 (N=21), 3 (N=3) or 4 cycles (N=16). All had active progressive disease at enrollment and no bridging therapy was given. The cords used for each of the 117 cycles were selected without consideration for HLA match, which was 0/6 (N=53), 1/6 (N=46), 2/6 (N=14), 3/6 (N=3) and 4/6 (N=1). The product infused consisted of >94% NK cells (expanded >2,700 fold), with only 0.02% T cells. The NK cells were 96% viable, 91% bound to AFM13, and 97.6% CD16+. The products were infused fresh. There was 1 grade 2 infusion-related reaction (IRR) in 115 infusions of AFM13-NK (0.8%) and 27 IRR infusion related reactions in 350 infusions of AFM13 (7.7%) (1 G3, 26 G2). We saw no cases of CRS, ICANS or GVHD of any grade. DL3 (108 NK/Kg) was established as the RP2D. The ORR and CR were 92.8% and 66.7%, respectively (94.4% and 72.2%, respectively, in 36 pts treated at the RP2D). Nine pts had a response consolidated with SCT (5 allo, 4 auto). At median follow-up of 14 (6-34) months, the EFS/OS rates are 31%/76%; median EFS/OS are 8 months/not reached. No relapses were associated with antigen loss. In the subset of pts planned for 4 cycles at RP2D, 15 of 23 pts remained event free at 6 mo (4 pts with and 11 without SCT consolidation), for 6-month EFS/OS rates of 65%/83%. Overall, 8 of the 9 pts (89%) receiving a consolidation SCT are event free at 10 to 34 months. AFM-bound CB NK cells were detected in blood from day 1 post infusion for up to 3 weeks. Donor NK levels followed similar patterns after each cycle, which argues against a sensitization after the first NK infusion. CyTOF studies showed increased expression of AFM13 and activation markers in donor NK cells. We confirmed trafficking of AFM13-bound donor NK cells to tumor sites shortly after infusion.
It can be concluded that CB-derived cytokine-induced memory-like NK cells precomplexed with AFM13 have excellent tolerability and activity for pts with heavily pretreated and refractory CD30+ lymphoma.
How to Easily View the Clinical Results Using Synapse Database?
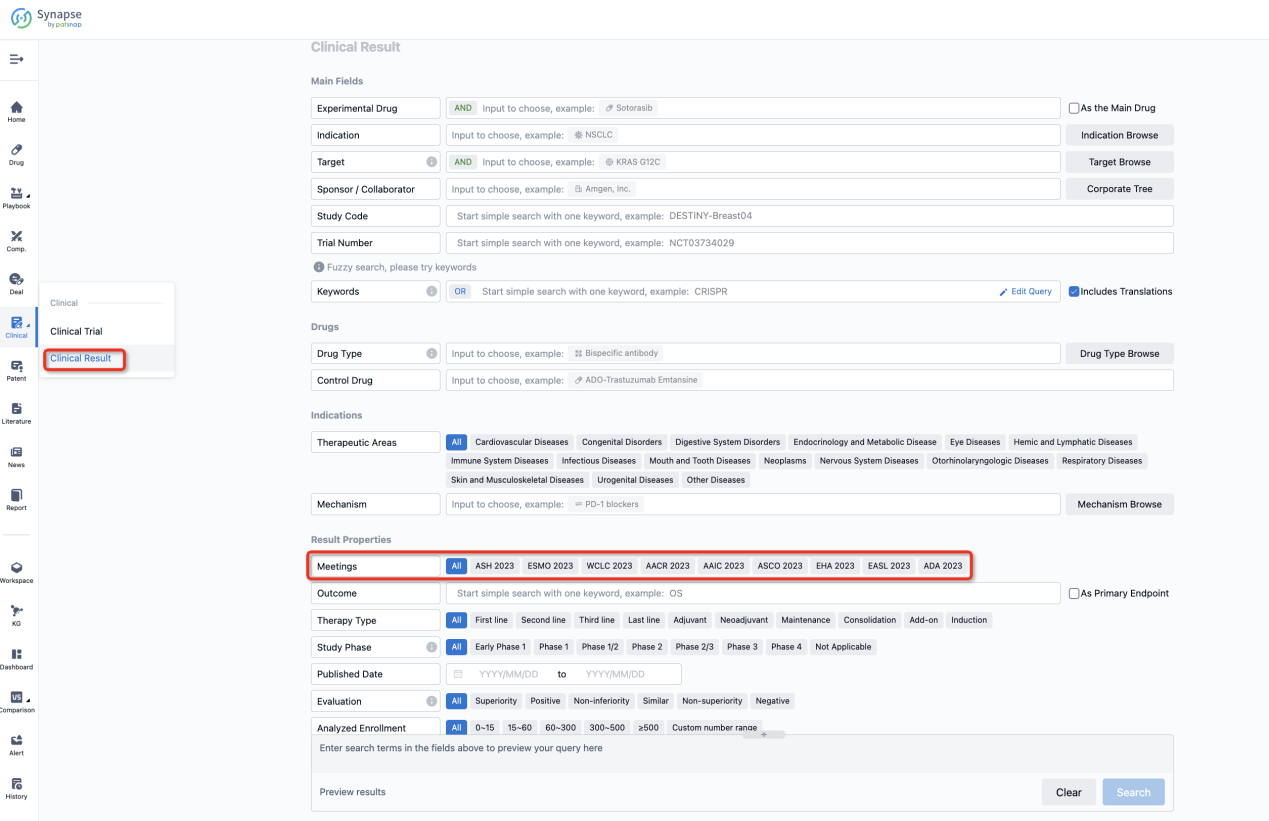
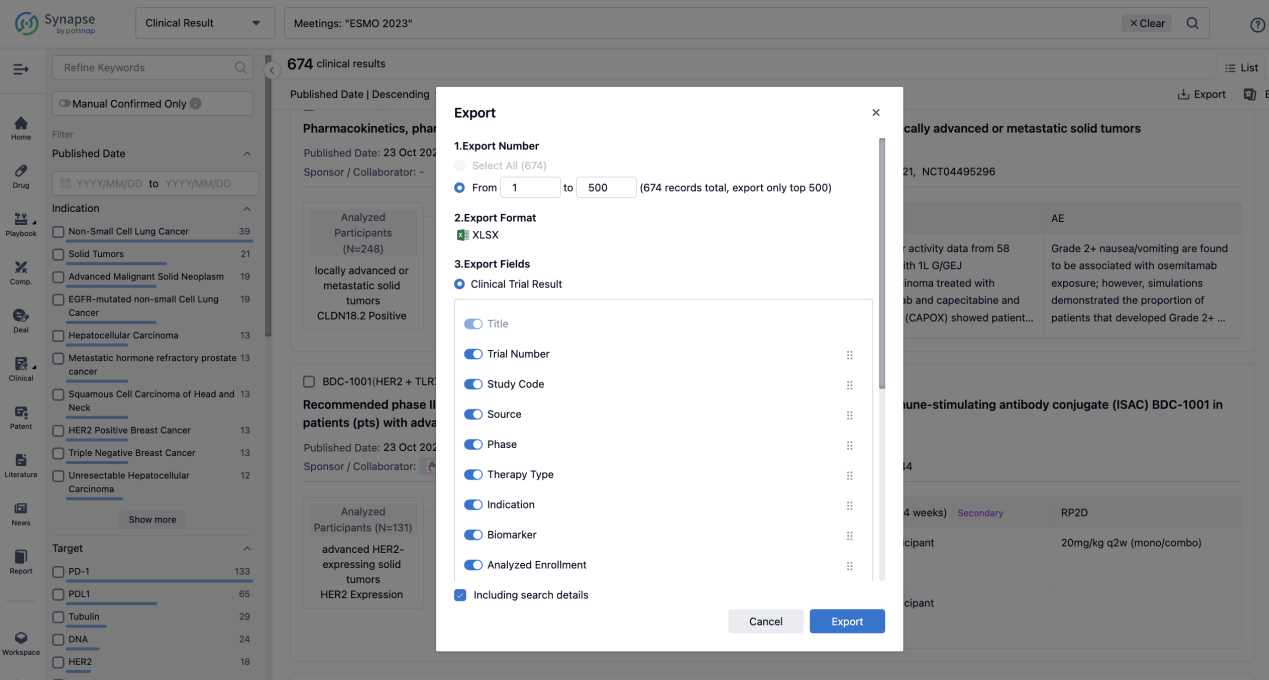
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
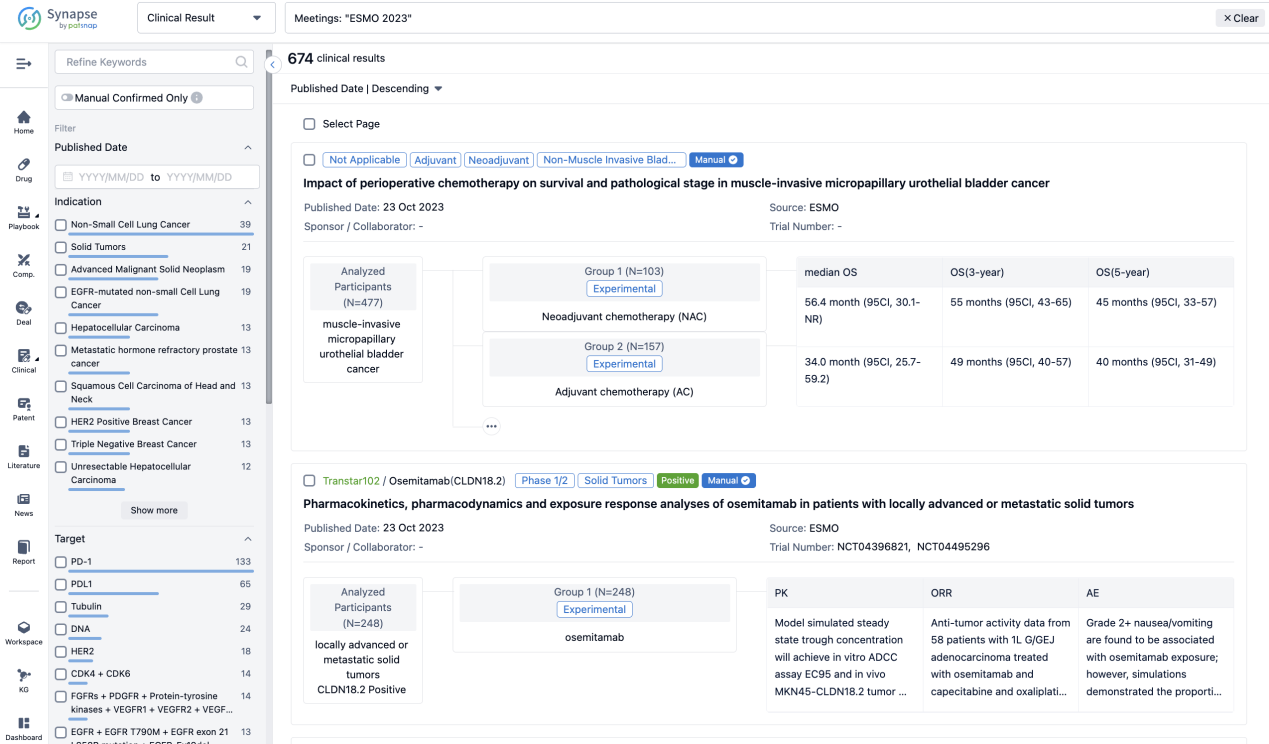
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!