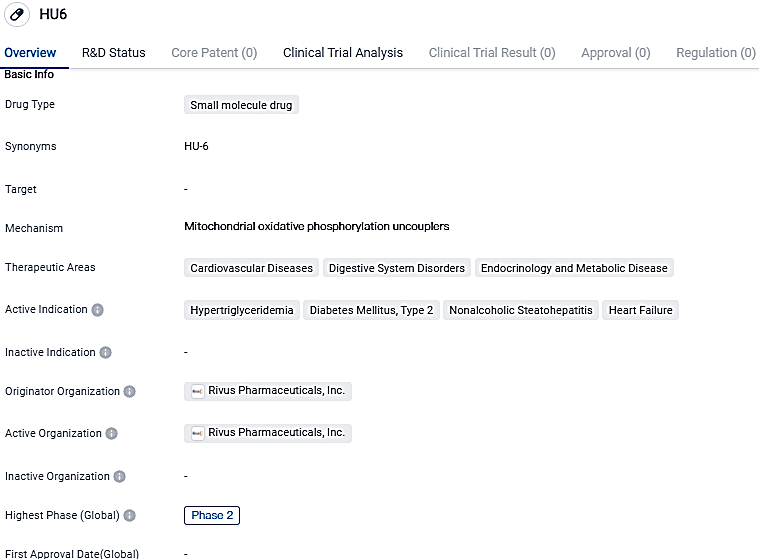
Rivus Pharmaceuticals' HU6 2a trial showed positive outcomes in treating high BMI individuals with Non-alcoholic Fatty Liver Disease
Rivus Pharmaceuticals Inc., a company in the clinical testing phase in the biopharmacology sector and specializing in enhancing cardiometabolic wellbeing, has disclosed in The Lancet Gastroenterology & Hepatology that they have published the findings of a Phase 2a metabolic research on HU6. HU6, a pioneering metabolic accelerator of the controlled category currently under examination, was studied in patients with nonalcoholic fatty liver disease (also known as metabolic dysfunction-associated fatty liver disease) and an above-average body mass index.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Findings demonstrated that HU6 notably diminished liver fat in contrast to the placebo in both the complete research group and among participants with an elevated risk of TypeTwo Diabetes. HU6 also cut down body weight without losing lean muscle mass and advanced metabolism and systemic inflammation indicators. HU6, the firm's front-line drug, is a phase 2 oral, controlled metabolic catalyst in clinical trials.
"The reassuring efficacy and safety outcomes of the Phase 2a metabolic clinical evaluation of HU6 boosts our belief that this pioneering treatment has the capacity to offer a potent, easy-to-take therapy for various cardiometabolic ailments linked with obesity," stated Mazen Noureddin, M.D., a hepatologist at Houston Research Institute and Houston Methodist Hospital, who was the lead writer of the publication.
Numerous investigational treatments have been examined in patients suffering from NAFLD/MASLD, but not many options are available that can accurately address this condition in obese patients with increased liver fat. The significant reductions in liver fat, along with larger than anticipated decreases in inflammatory markers, all make HU6 a great potential fresh approach," Mazen Noureddin, M.D. further added.
Jayson Dallas, M.D., the chief executive officer of Rivus Pharmaceuticals, noted, "There are more and more indications that HU6 can enhance metabolism in a harmless and regulated way that allows fat-specific weight drop while diminishing inflammation. We are extremely hopeful about the Phase 2a metabolic study outcomes and are persistently assessing HU6 broadly to enhance cardiometabolic wellbeing in persons struggling with obesity."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, targets, organizations, clinical trials, clinical results, and drug patents related to this indication.
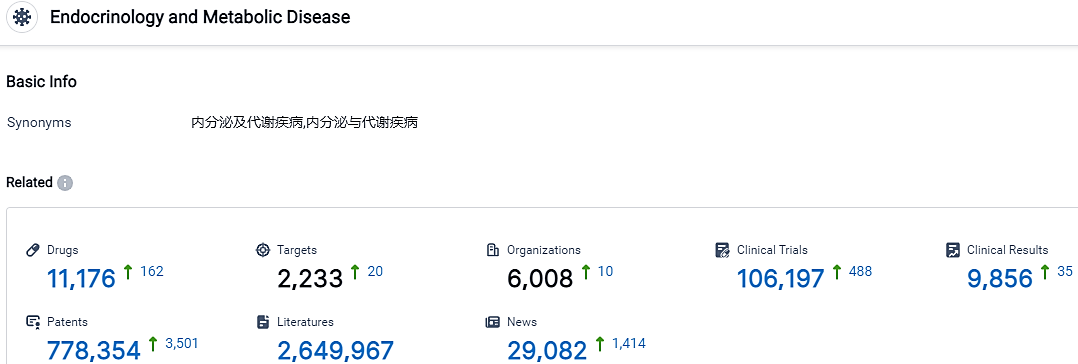
According to the data provided by the Synapse Database, As of October 17, 2023, there are 11176 investigational drugs for the endocrinology and metabolic disease, including 2233 targets, 6008 R&D institutions involved, with related clinical trials reaching 106197,and as many as 778354 patents.
HU6, ranks as the most progressive CMA currently in the clinical trial stage. It is meticulously engineered to regulate both absorption and metabolism. The unique methodology of HU6 augments the resting metabolic rate of the body, devoid of any recognizable or real elevation in body temperature. Rivus is actively running clinical research schemes for HU6, targeting illnesses like heart failure with maintained ejection fraction, Metabolic dysfunction related steatohepatitis (MASH), Type 2 diabetes, and obesity.