Roche introduces Alnylam's hypertension RNAi therapy, Zilebesiran, with a potential transaction value of up to 2.8 billion dollars
On July 24, 2023, Alnylam announced that it has reached a strategic agreement with Roche to develop and commercialize Zilebesiran, Alnylam's RNAi therapeutic drug for the treatment of hypertension. Under the terms of the agreement, Alnylam will receive a $310 million upfront payment and is eligible for additional payments, including development milestone payments over the next several years, as well as regulatory and sales milestone payments, potentially totaling up to $2.8 billion in transaction value.
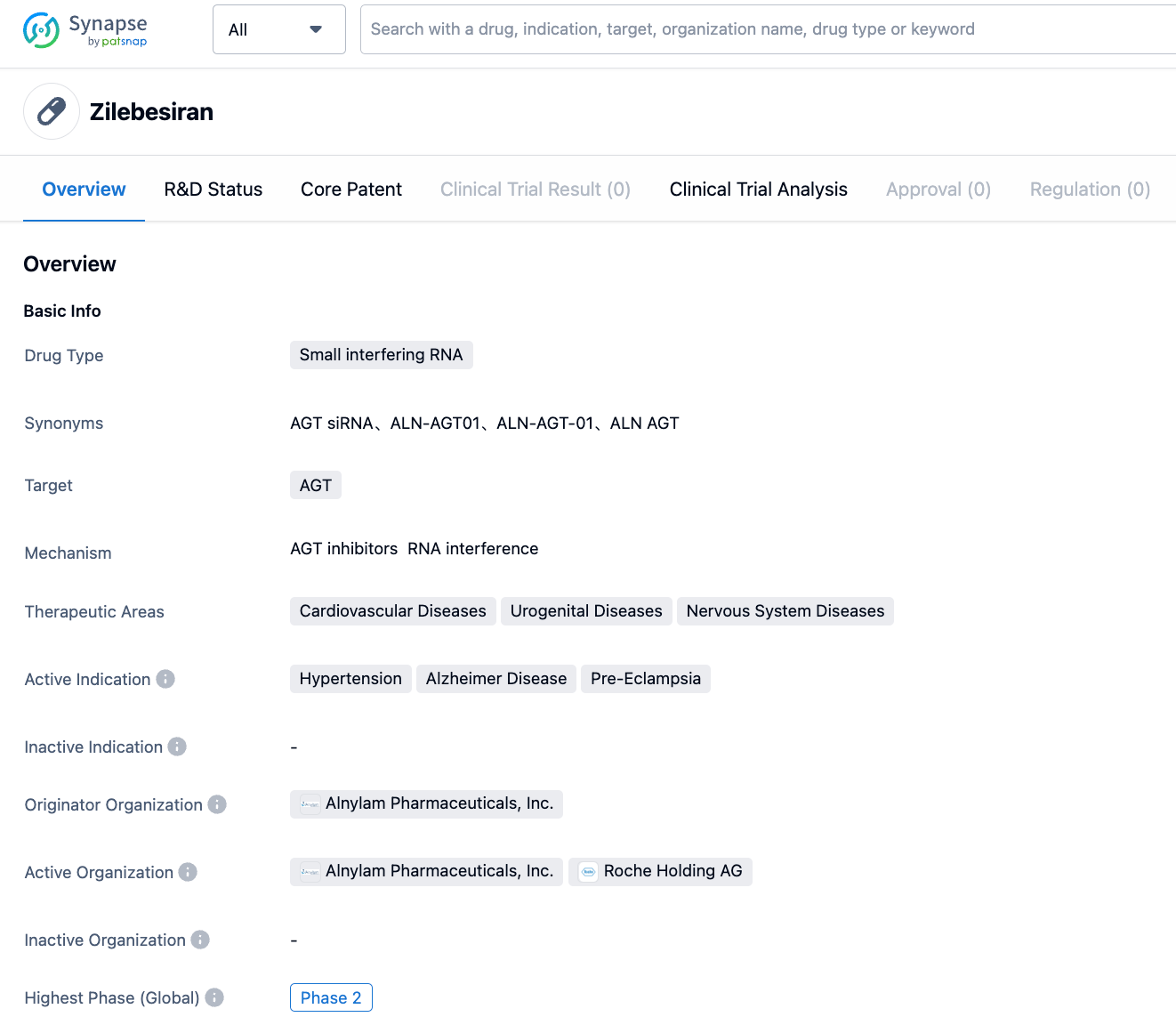
Zilebesiran is an RNAi drug developed by Alnylam that targets liver-expressed angiotensinogen (AGT), and is currently in Phase II development. Zilebesiran is comprised of a small interfering RNA (siRNA) covalently linked to an N-acetyl galactosamine (GalNAc) ligand, which can bind specifically to protein receptors expressed on the surface of liver cells, thus achieving precise delivery. Angiotensinogen is the unique precursor to angiotensin peptides and plays a key role in the pathogenesis of hypertension. Zilebesiran significantly reduces the production of AGT in liver cells, and achieves long-lasting effects through the repetitive recycling of the RNA interference silencing complex (RISC).
On July 19, 2023, the results of Phase I study of Zilebesiran for the treatment of hypertension were officially published in the New England Journal of Medicine (NEJM). The key data reported in this paper showed that in Phase I study, compared with placebo, Zilebesiran was associated with dose-dependent reductions in serum AGT. After a single dose of ≥200mg of Zilebesiran, a sustained and prolonged reduction in blood pressure was achieved within a 24-hour cycle, and the effect lasted for up to 6 months. Alnylam is currently evaluating the safety and efficacy of Zilebesiran in the KARDIA Phase II clinical program, including as monotherapy (KARDIA-1), and in combination with standard antihypertensive drugs (KARDIA-2). The company expects to report the results of these trials by mid-2023 and the end of the year. Zilebesiran can be regarded as a milestone drug in the field of hypertension. However, due to the small scale and short duration of the trial, more safety and efficacy data are required to support the later development of the drug.
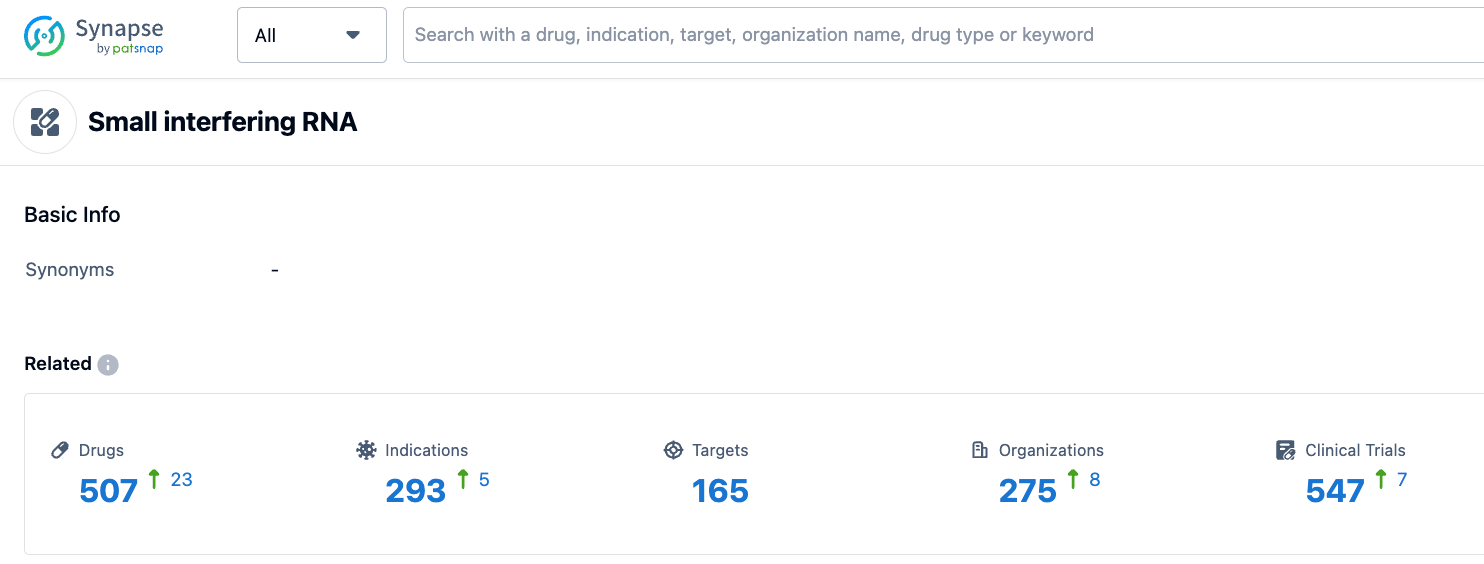
According to the information disclosed by Synapse (click on the image below to directly reach the siRNA drug type, and after registration and login you can obtain detailed information about the investigational drugs, indications, targets, research institutions, clinical trials, etc. under this drug type for free), as of July 25, 2023, there are a total of 507 investigational drugs under the siRNA drug type, including 293 types of indications, 165 targets, 275 research institutions, and 547 related clinical trials. We look forward to the smooth follow-up development of Zilebesiran, providing a new treatment option for patients with high blood pressure.