An Overview of Sun Pharmaceutical 's 159 Drug Pipelines ——Top 50 Pharmaceutical Companies R&D Progress
Sun Pharmaceutical Industries Ltd. is a pharmaceutical company based in Maharashtra, India. It was founded in 1983 and has since become one of the largest pharmaceutical companies in India. The company operates in the field of biomedicine and focuses on the development and manufacturing of generic and branded drugs.
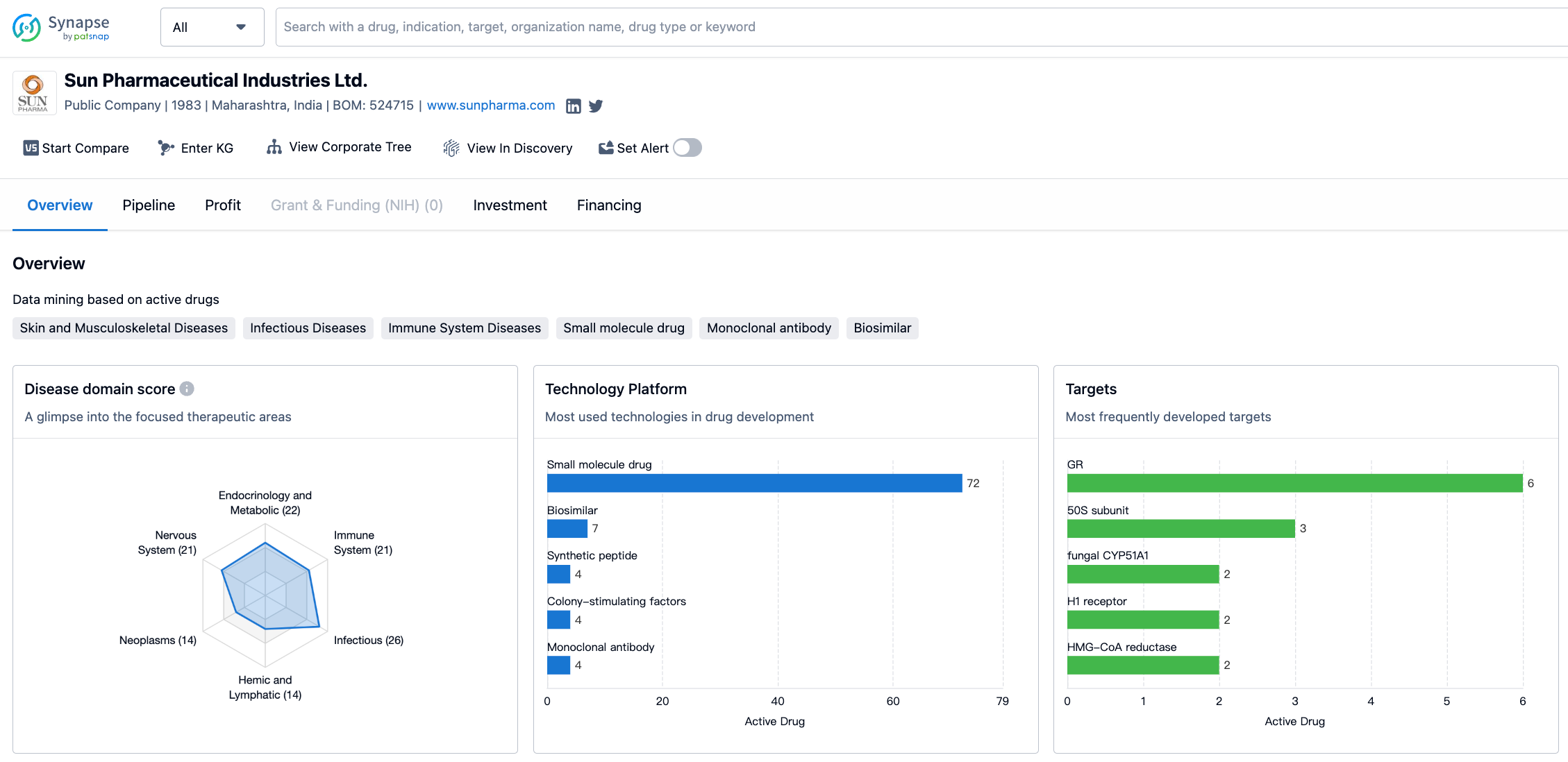
In terms of therapeutic areas, Sun Pharmaceutical Industries Ltd. has a diverse portfolio. The company has developed drugs for a wide range of diseases and conditions. The largest distribution of drugs is in the area of skin and musculoskeletal diseases, with a total of 41 drugs. This is followed by infectious diseases with 26 drugs, and other diseases with 22 drugs. Endocrinology and metabolic diseases, immune system diseases, and urogenital diseases each have 22 drugs developed by the company. Nervous system diseases, respiratory diseases, hemic and lymphatic diseases, and neoplasms each have between 14 and 20 drugs developed by Sun Pharmaceutical Industries Ltd. Digestive system disorders, congenital disorders, cardiovascular diseases, otorhinolaryngologic diseases, eye diseases, and mouth and tooth diseases have a lower number of drugs developed, ranging from 7 to 12.
When it comes to the most frequently developed targets by Sun Pharmaceutical Industries Ltd., the company has focused on a variety of molecular targets. The most frequently developed target is GR (glucocorticoid receptor), with 5 drugs developed. This is followed by the 50S subunit, fungal CYP51A1, H1 receptor, HMG-CoA reductase, COX (cyclooxygenase), and CSF-3R (colony-stimulating factor 3 receptor), each with 2 drugs developed. Other targets such as CaN (calcineurin), PTGFR (prostaglandin F receptor), SQLE (squalene epoxidase), DNMT1 (DNA methyltransferase 1), ODC (ornithine decarboxylase), RARs (retinoic acid receptors), IL-2R (interleukin-2 receptor), 5-LOX + α4β1 (5-lipoxygenase and α4β1 integrin), free radicals + RARs, SCNA (sodium channels), ER (estrogen receptor), and ACE (angiotensin-converting enzyme) have 1 drug developed by the company.
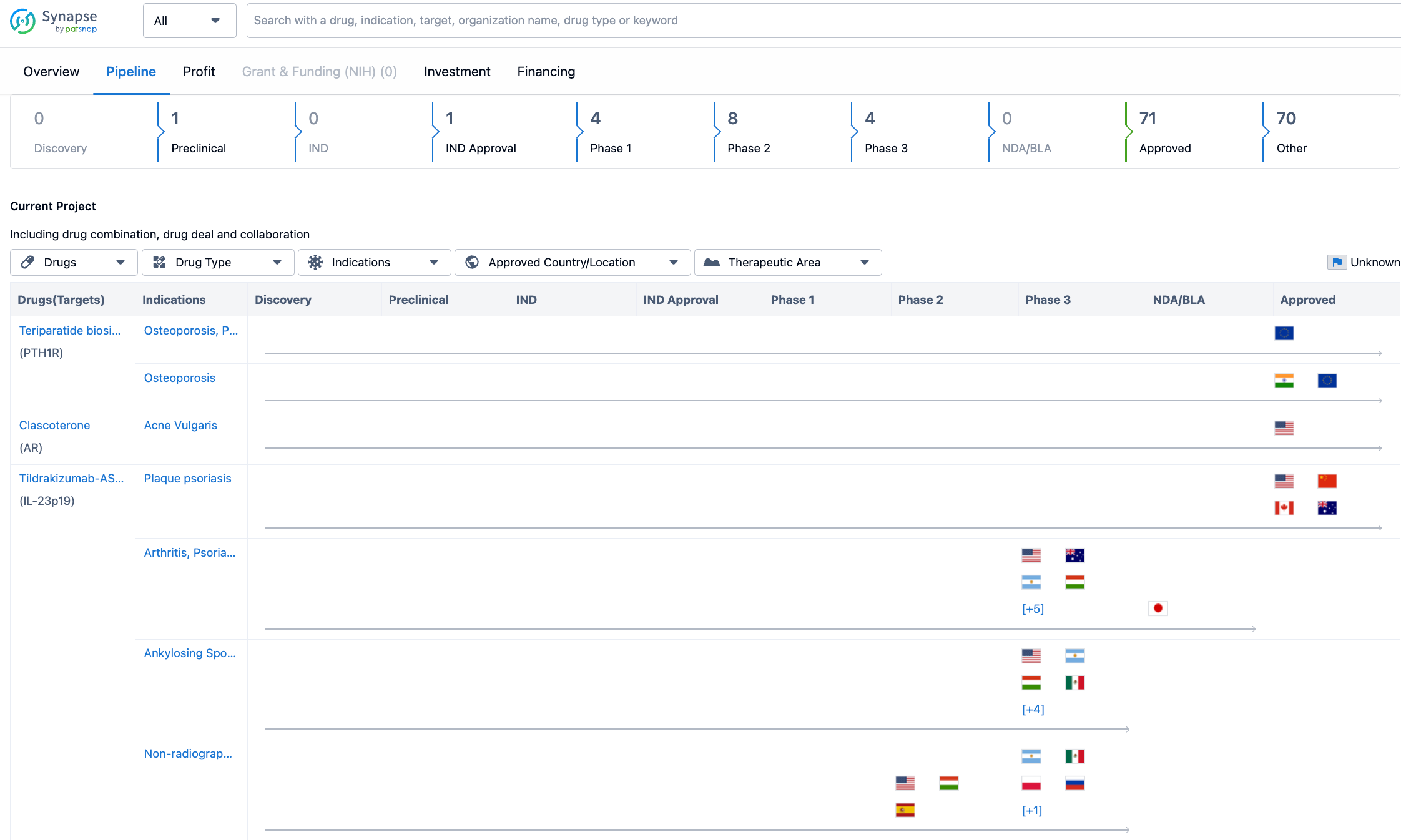
In terms of the pipeline, Sun Pharmaceutical Industries Ltd. has a significant number of drugs in various stages of development. As of the latest update, the company has 1 drug in the preclinical stage, 1 drug in the IND (Investigational New Drug) approval stage, 4 drugs in Phase 1, 8 drugs in Phase 2, and 4 drugs in Phase 3. However, Sun Pharmaceutical Industries Ltd. has a substantial number of drugs that have already been approved, with a total of 71 drugs. Additionally, the company has 70 drugs in other stages of development.
Sun Pharmaceutical Industries Ltd. has established itself as a major player in the pharmaceutical industry, particularly in the field of biomedicine. With a wide range of therapeutic areas covered and a diverse portfolio of drugs, the company has demonstrated its commitment to addressing various diseases and conditions. The focus on different molecular targets further highlights the company's dedication to developing innovative and effective treatments. The pipeline of drugs in various stages of development indicates that Sun Pharmaceutical Industries Ltd. is actively working on bringing new drugs to the market. Overall, the company's track record and ongoing efforts position it as a key player in the pharmaceutical industry, contributing to advancements in biomedicine and improving patient outcomes.