RUBY Study: Phase III Success Prompts Broadened Use of Jemperli in Advanced Uterine Cancer
GSK plc has disclosed pivotal findings demonstrating both a considerable increase in overall survival from the first segment and enhanced progression-free survival metrics from the second segment of the comprehensive phase III RUBY/ENGOT-EN6/GOG3031/NSGO trial. These findings pertain to adult individuals who are battling either initial high-stage or relapsed endometrial carcinoma.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
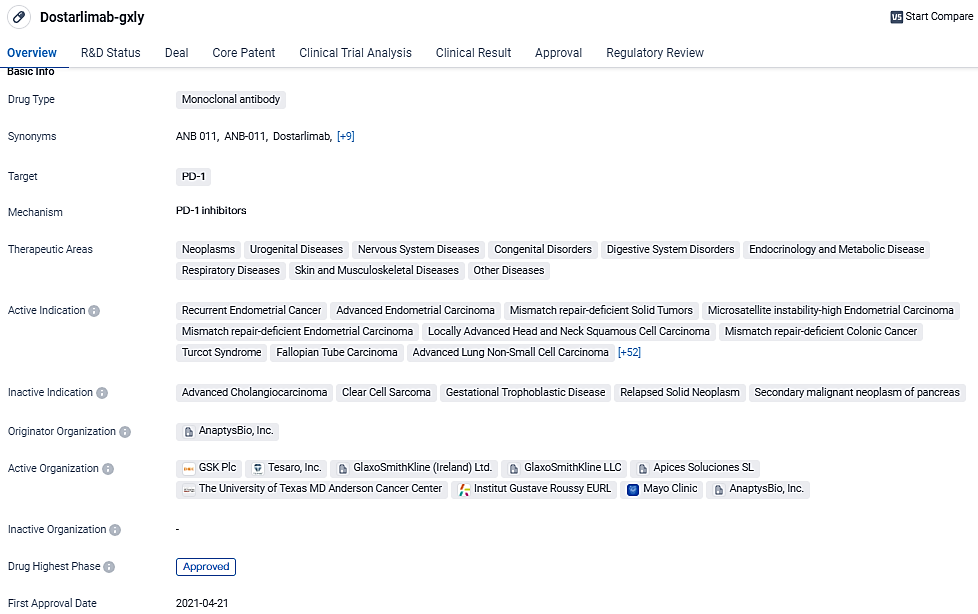 The objective of the RUBY phase III clinical study is to ascertain the subset of patients with advanced initial or recurring endometrial carcinoma who might derive an advantage from the therapeutic combination of Jemperli (dostarlimab-gxly) and chemotherapy, optionally followed by Zejula (niraparib) as a maintenance therapy.
The objective of the RUBY phase III clinical study is to ascertain the subset of patients with advanced initial or recurring endometrial carcinoma who might derive an advantage from the therapeutic combination of Jemperli (dostarlimab-gxly) and chemotherapy, optionally followed by Zejula (niraparib) as a maintenance therapy.
The initial segment of the RUBY phase III investigation is examining the effects of combining dostarlimab-gxly with the current chemotherapy standard, then continuing with dostarlimab-gxly mono-therapy, in contrast to the use of placebo alongside chemotherapy, followed by a placebo maintenance schedule. The subsequent division of the RUBY phase III assessment is focusing on the efficacy of dostarlimab-gxly with standard chemotherapy, subsequently proceeding with the addition of dostarlimab-gxly and niraparib as a sustained therapy, as opposed to a regimen of placebo coupled with chemotherapy, followed by long-term placebo.
The compatibility and adverse effect rates of the treatment combinations—including dostarlimab-gxly with carboplatin-paclitaxel and dostarlimab-gxly with carboplatin-paclitaxel succeeded by the dostarlimab-gxly and niraparib protocol—were found to align with the pre-established safety outlines of each drug when administered separately.
The presented findings provide further evidence suggesting the enhanced efficacy of the combination treatment using dostarlimab-gxly and chemotherapy, with or minus the continued use of niraparib. This is significant for the broader collective of those facing primary advanced or recurring endometrial carcinoma. This includes those diagnosed with mismatch repair proficient (MMRp)/microsatellite stable malignancies—a group for whom no immuno-therapy-centric treatments currently obtain regulatory clearance.
In a comment from Hesham Abdullah, Senior Vice President and Global Head of Oncology R&D at GSK, he noted: "The insights we've gathered thus far add to the accumulating body of knowledge endorsing the use of dostarlimab-gxly as a central component in our pursuits within immuno-oncology research. It remains our dedicated mission to find innovative combinations and applications for dostarlimab-gxly, as a solitary treatment or paired with other modalities, seeking to elevate the standards of care for those battling cancers with few existing therapeutic alternatives."
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
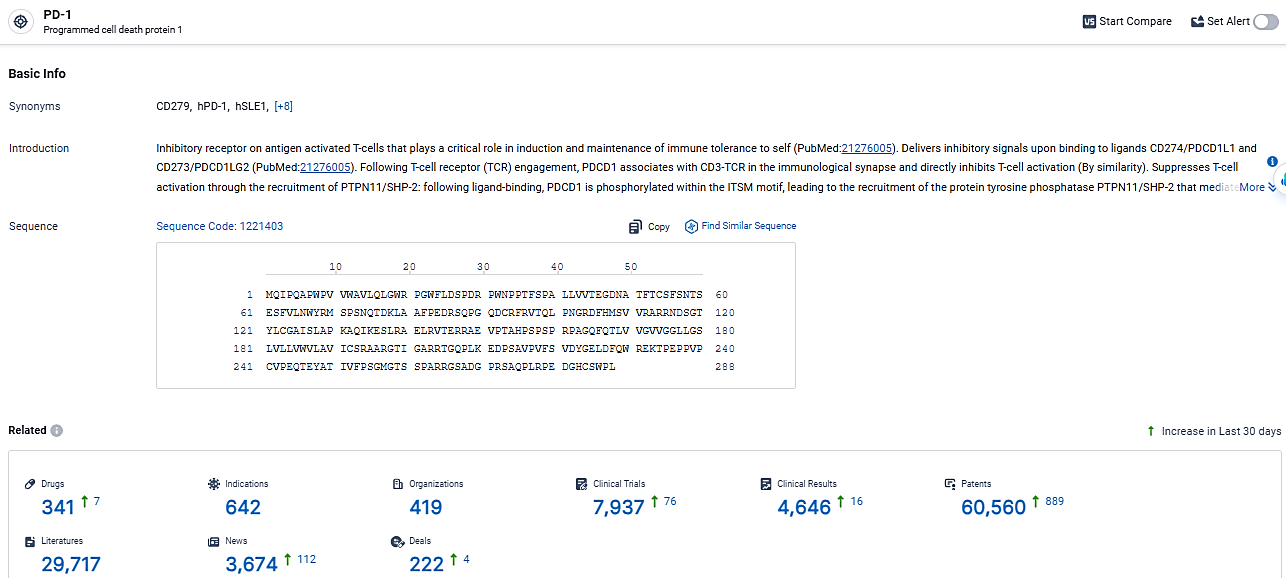
According to the data provided by the Synapse Database, As of March 19, 2024, there are 341 investigational drugs for the PD-1 target, including 642 indications, 419 R&D institutions involved, with related clinical trials reaching 7937, and as many as 60560 patents.
Dostarlimab-gxly is a monoclonal antibody drug that targets PD-1 and has been approved for the treatment of various cancers and other diseases. Its approval in multiple countries and the designations it has received highlight its potential as a promising therapeutic option in the field of biomedicine.