Sage Therapeutics Reveals Phase 2 Results of Dalzanemdor (SAGE-718) in Alzheimer’s Cognitive Impairment and Dementia
Sage Therapeutics, Inc. (Nasdaq: SAGE) has released preliminary findings from its LIGHTWAVE trial, a 12-week Phase 2 study that was randomized, double-blind, and placebo-controlled. This study aimed to assess the impact of dalzanemdor (SAGE-718) on individuals with mild cognitive impairment (MCI) or mild Alzheimer’s Disease (AD) dementia. The LIGHTWAVE trial did not find a statistically significant change from baseline in participants receiving dalzanemdor compared to those on placebo, as measured by the Wechsler Adult Intelligence Scale Fourth Edition (WAIS-IV) Coding Test scores at Day 84, which was the primary metric for the study.
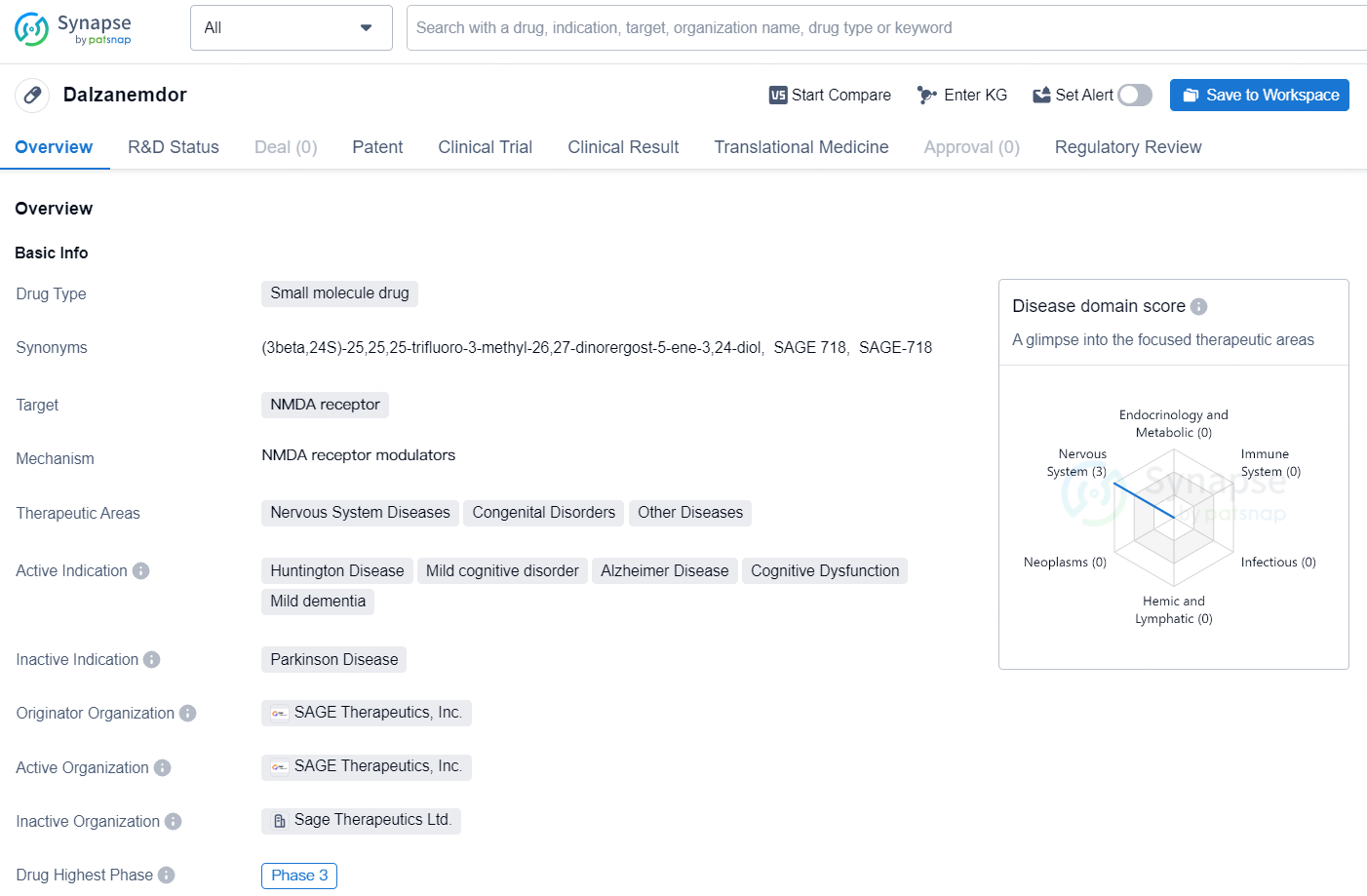
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
According to the gathered data, the Company has decided against pursuing additional clinical development of dalzanemdor for Alzheimer's Disease (AD). They anticipate releasing topline results from the Phase 2 DIMENSION Study involving dalzanemdor in individuals with cognitive deficits linked to Huntington’s Disease later this year.
“Alzheimer’s Disease presents a highly intricate and distressing challenge, with individuals experiencing mild cognitive impairment and mild dementia requiring more treatment alternatives. While we are disheartened by the outcomes of the LIGHTWAVE Study, we appreciate the contributions of participants, researchers, caregivers, patient advocates, and the Alzheimer's community that enabled this crucial research. We hope our findings will guide future studies,” stated Barry Greene, the Chief Executive Officer of Sage Therapeutics.
Results of the LIGHTWAVE Study
The LIGHTWAVE study was a 12-week, Phase 2 randomized, double-blind, placebo-controlled trial aimed at assessing the impact of dalzanemdor on individuals with mild cognitive impairment (MCI) or mild dementia related to AD. In total, 174 individuals were assigned to the study.
The LIGHTWAVE trial did not reveal a statistically significant difference from baseline between the participants receiving dalzanemdor and those taking a placebo, as measured by the WAIS-IV Coding Test score after 84 days.
Overall, dalzanemdor was well-received, with no new safety concerns identified. Most treatment-related adverse events were classified as mild to moderate in intensity. Analyses indicated that there were no significant variations in exploratory endpoints, such as the RBANS total score or the MoCA total score, between the group treated with dalzanemdor and the placebo group.
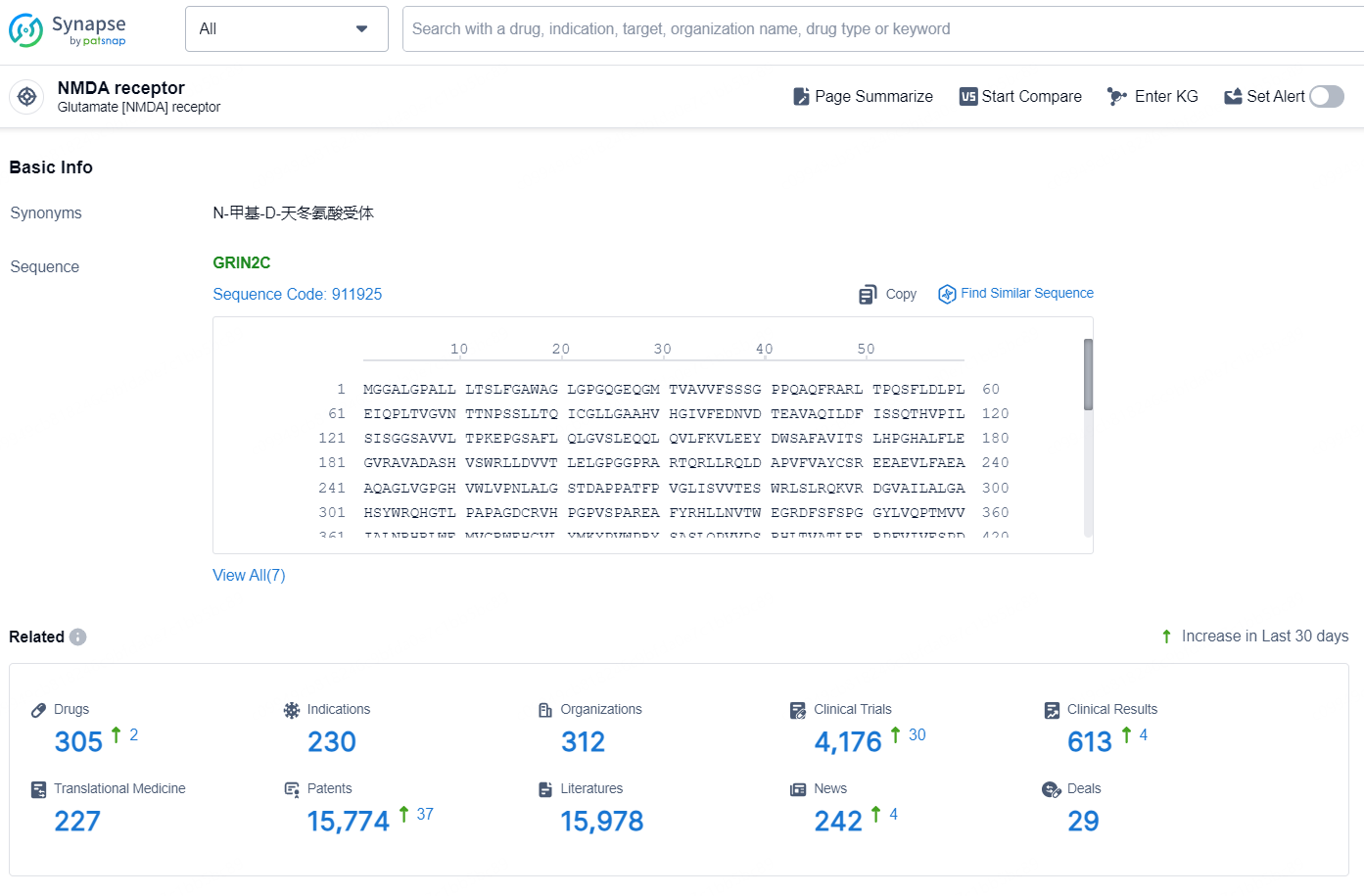
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of October 11, 2024, there are 305 investigational drugs for the NMDA target, including 230 indications, 312 R&D institutions involved, with related clinical trials reaching 4176, and as many as 15774 patents.
Dalzanemdor is a small molecule drug developed by SAGE Therapeutics, Inc. The drug targets the NMDA receptor and is being developed for the treatment of various nervous system diseases, congenital disorders, and other diseases. The drug is currently in Phase 3 of clinical trials, indicating that it has advanced to a late stage of development.