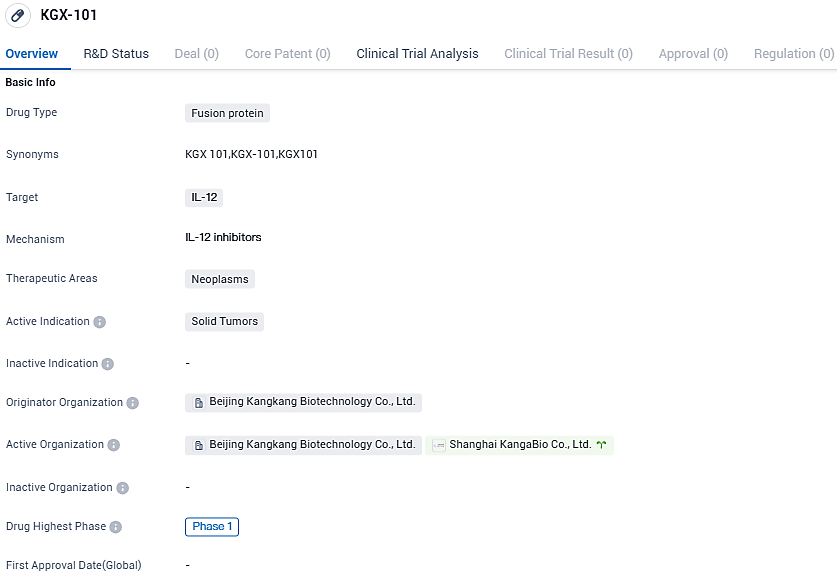
Sanyou Bio conveys hearty congratulations to KangaBio on securing IND endorsement for their modern IL-12 Prodrug cancer immunotherapy
KangaBio made a declaration on 26th of October 2023, that their independent research and development clinical trial application for KGX101 has received formal sanction from the FDA in the United States. KGX101 is a fusion protein, which has been recombined with IL-12 Fc and is intended for use via an intravenous injection. The clinical testing for KGX101 is set to take place concurrently in Australia and the United States.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
These trials are mostly focused on advanced solid tumors, and the effectiveness will be assessed either alone, or in combination with anti-PD-L1 antibodies. Several Australian research institutions have already begun screening patients for participation in these clinical trials.
Sanyou Bio extends its congrats to our associate, KangaBio, for achieving this significant benchmark. This accomplishment bolsters our 2022 strategic alliance, which is committed to progressing the development and invention of antibody-based medications. Sanyou Bio feels satisfied to have participated in KangaBio's preclinical efficacy evaluations for KGX101, showcasing encouraging in vivo anti-tumor efficacy and safety results. We are eager to gain further understanding of its performance within the clinical phase.
KGX101 is a prodrug of Interleukin-12, produced using KangaBio's proprotein technology platform. The use of the antibody Fc region results in a prolonged half-life for KGX101. KGX101 becomes active in tumors specifically by joining the protease-cleavable linker that focuses on tumors, thus reducing systemic cytokine toxicity when the linker is divided by tumor-specific matrix metalloproteinases within the tumor environment. KGX101, like IL-12, triggers immune cells within the tumor, reforming the tumor environment for therapeutic advantages.
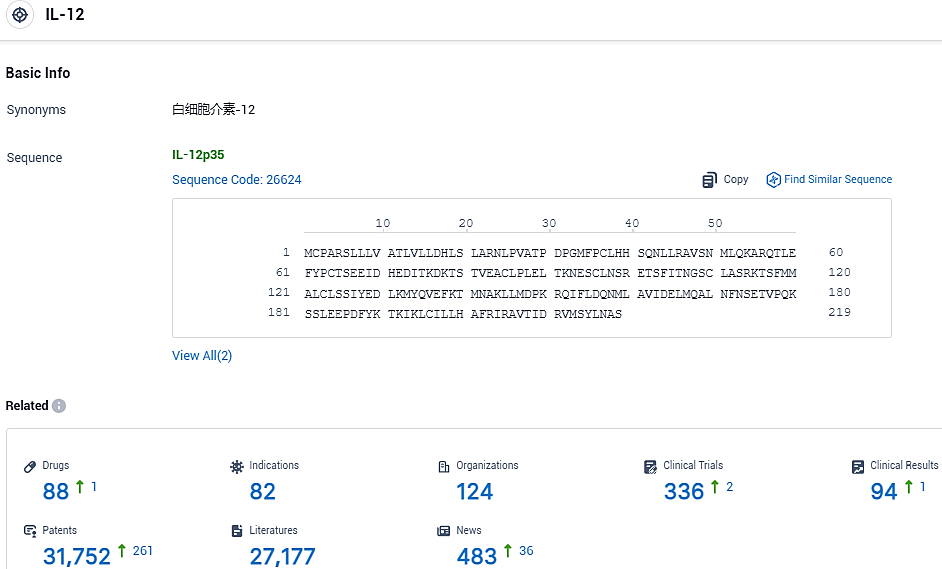
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 12, 2023, there are 88 investigational drugs for the IL-12 target, including 82 indications, 124 R&D institutions involved, with related clinical trials reaching 336, and as many as 31752 patents.
Currently, KGX-101 is in Phase 1 globally and in the preclinical stage in China. The drug's development stage and therapeutic focus indicate its potential in addressing the unmet medical need in the field of neoplasms, specifically solid tumors.