CBP-501: Brief Review of its R&D progress and the clinical outcome in 2023 ESMO
Metastatic pancreatic adenocarcinoma (PDAC) is an aggressive disease without third line standard-of-care treatment option. However, the latest research trial study of CBP-501 for the treatment of metastatic PDAC reported at the ESMO Congress exhibits promising therapeutic benefits.
CBP-501's R&D Progress
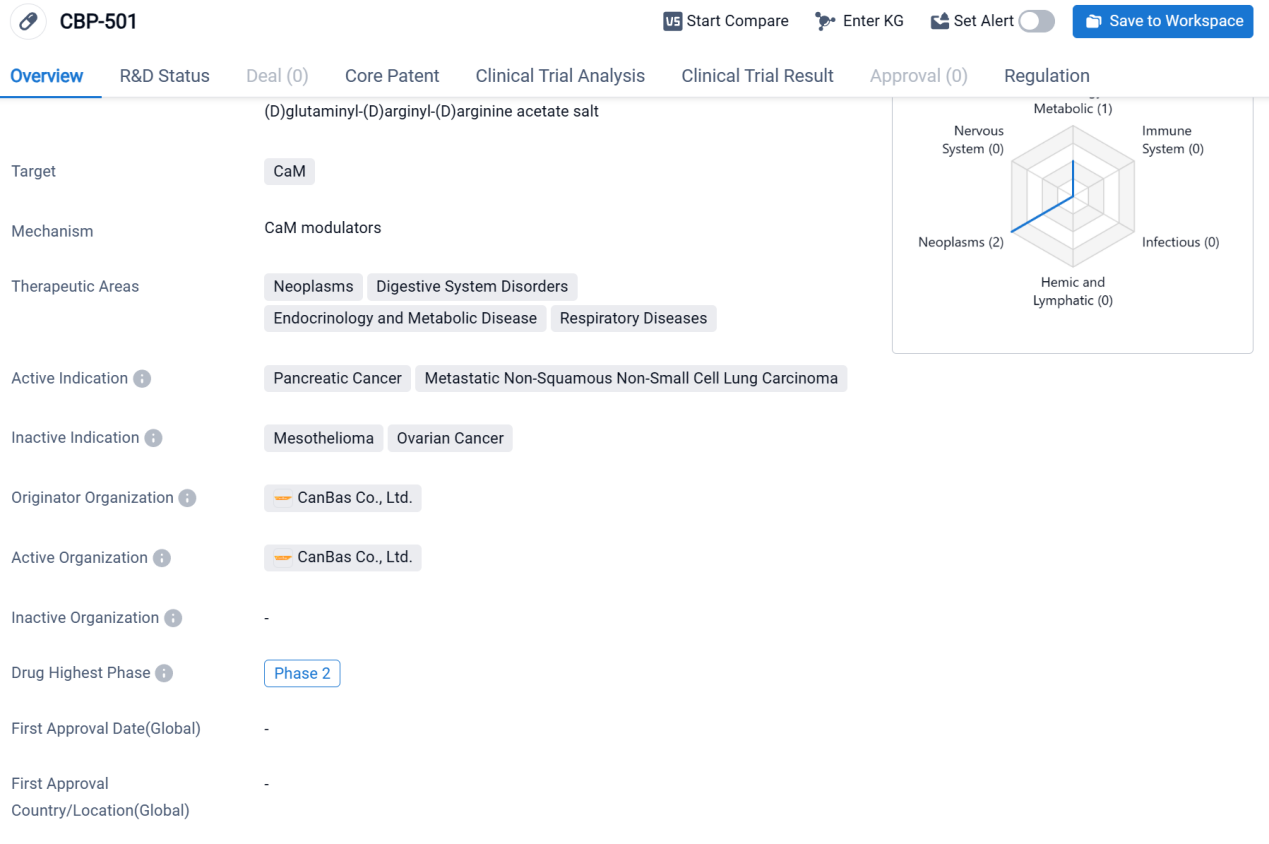
CBP-501 is a synthetic peptide drug that targets CaM (Calmodulin). It is being developed by CanBas Co., Ltd., an originator organization in the pharmaceutical industry.
CBP-501 has shown potential therapeutic applications in various therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic diseases, and respiratory diseases. Specifically, it has demonstrated efficacy in treating pancreatic cancer and metastatic non-squamous non-small cell lung carcinoma.
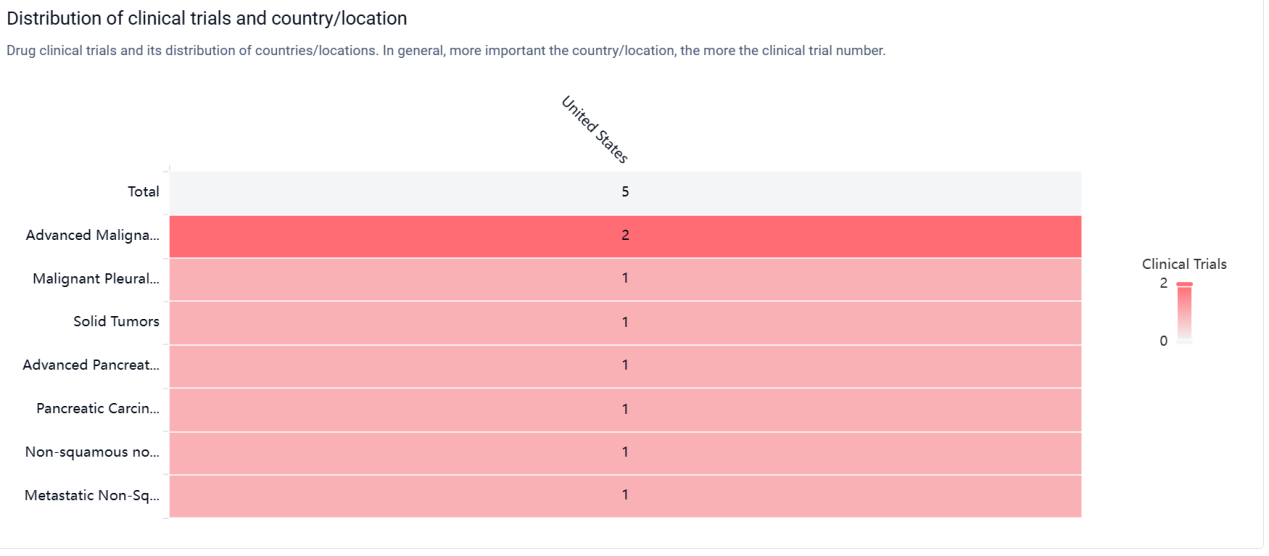
According to the Patsnap Synapse, the drug is currently in Phase 2, which is the highest phase of clinical development globally. And the clinical trial distribution for CBP-501 are primarily in the United States. The key indication is Advanced Malignant Solid Neoplasm.
Detailed Clinical Outcome of CBP-501
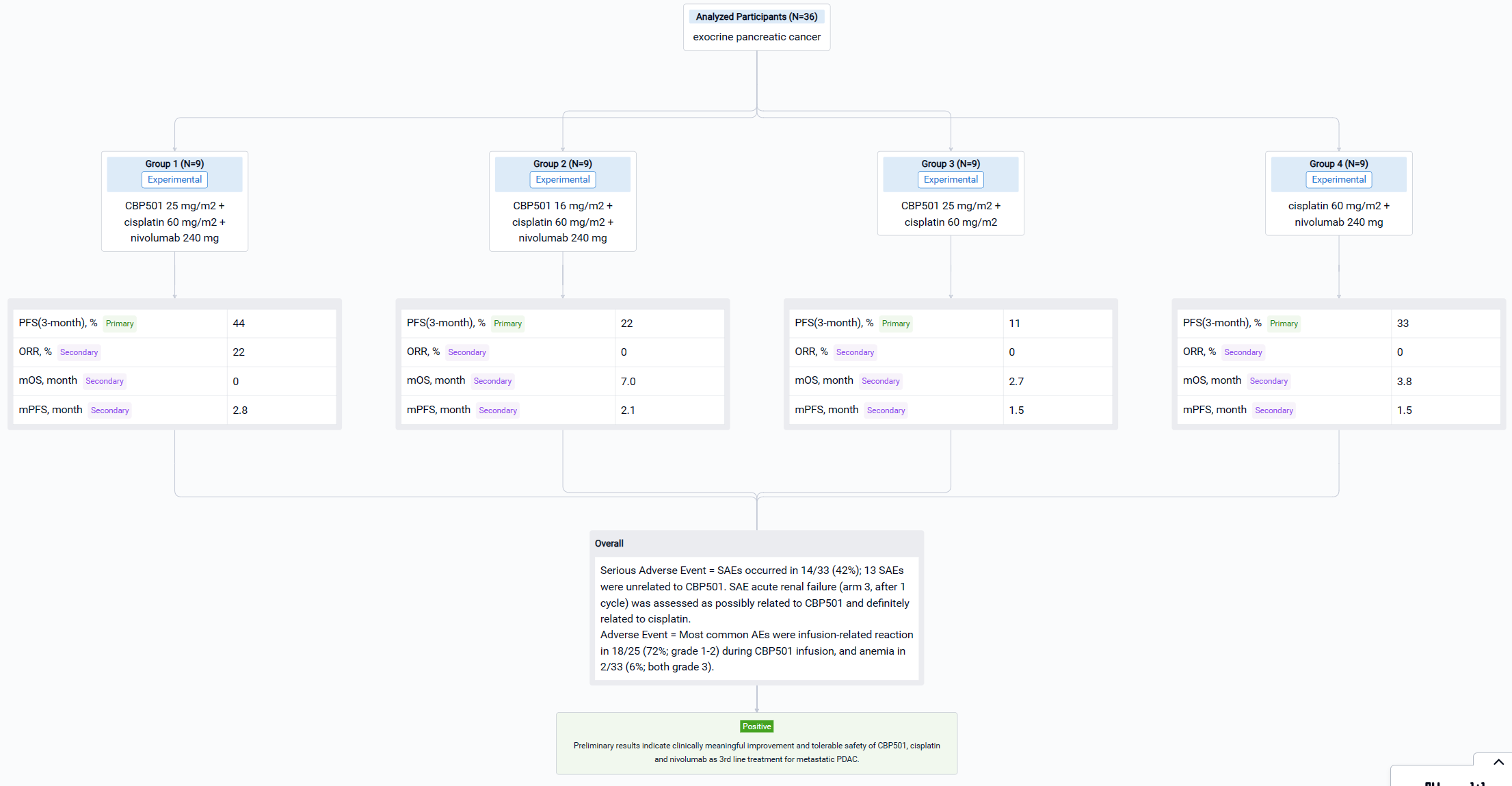
The randomized, parallel assignment, open-labeled clinical trial (NCT04953962) was aimed to establish the efficacy and safety of CBP501, cisplatin, and nivolumab for ≥ third-line treatment of patients with exocrine pancreatic cancer and WBC <10,000/mm3.
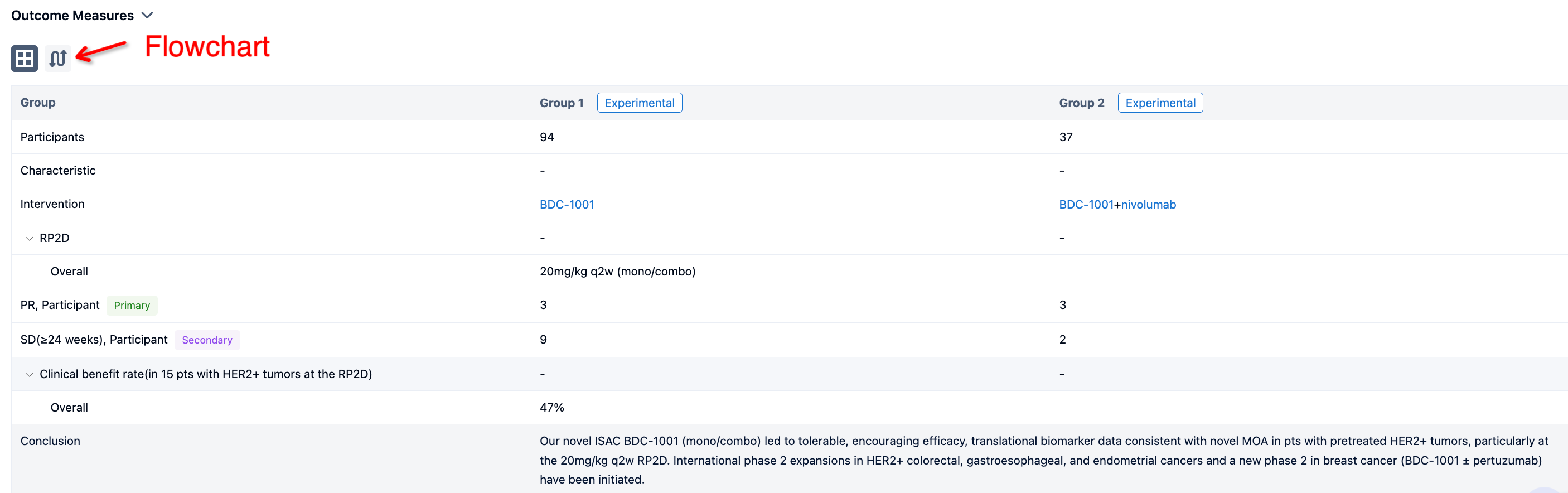
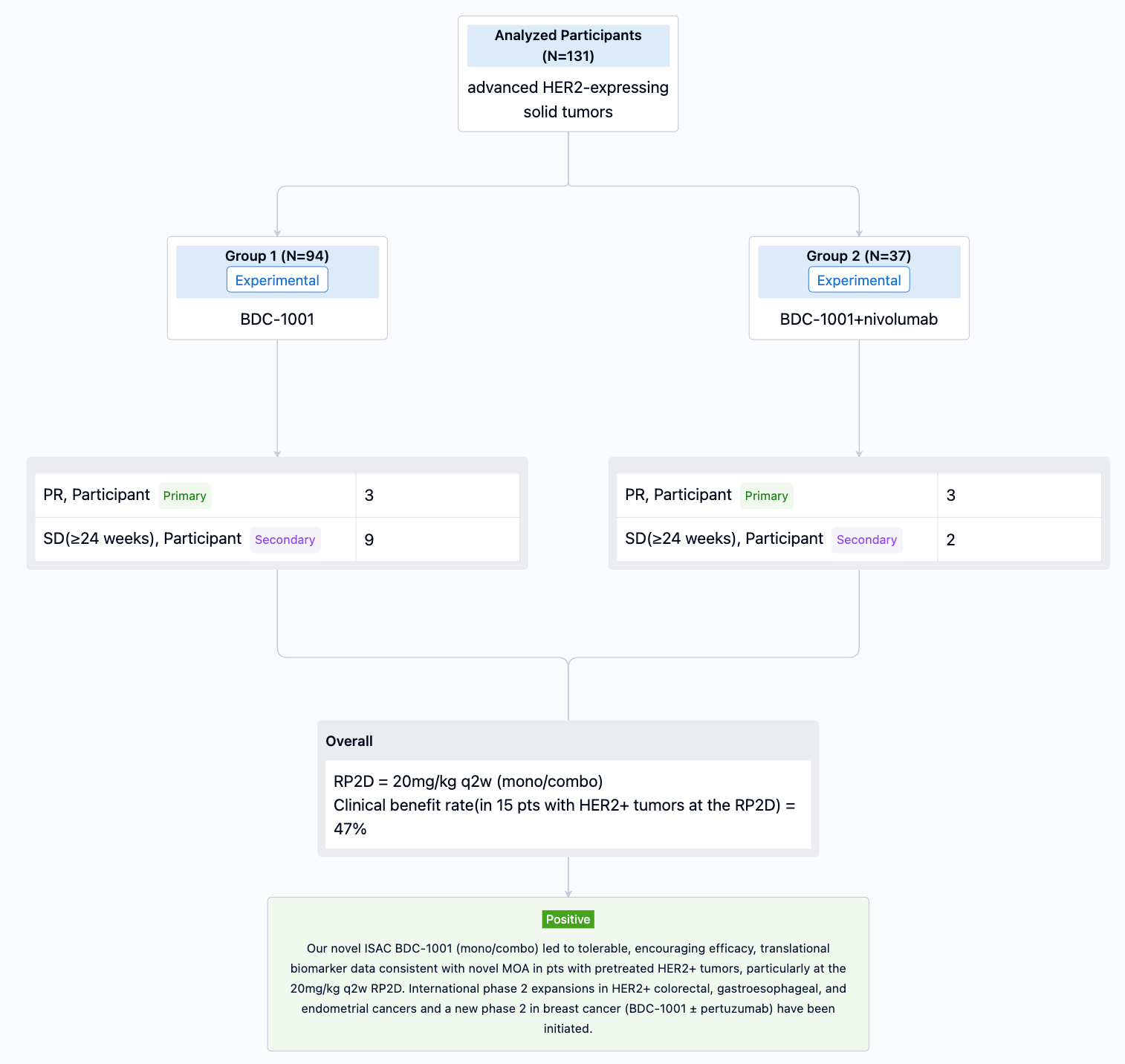
In this study, patients with metastatic PDAC, who failed two lines of systemic therapy, with white blood cell count <10,000/mm3 were stratified by ECOG (0 vs 1) and liver metastasis (present vs absent) and randomized 1:1:1:1 to arm 1 - CBP501 25 mg/m2 + cisplatin 60 mg/m2 + nivolumab 240 mg; arm 2 - CBP501 16 mg/m2 + cisplatin 60 mg/m2 + nivolumab 240 mg; arm 3 - CBP501 25 mg/m2 + cisplatin 60 mg/m2; or arm 4 - cisplatin 60 mg/m2 + nivolumab 240 mg. Patients received 4 cycles of combination therapy then 6 cycles of nivolumab every 21 days if without disease progression (nivolumab arms only). The primary endpoint was 3-month progression-free survival rate (3M PFSR) in the ITT population. Secondary endpoints were safety, PFS, objective response rate (ORR) and overall survival (OS).
The result showed that as of October 17, 2022, efficacy was evaluable in 36 patients. 3M PFSR were 44%, 22%, 11% and 33% in arms 1, 2, 3 and 4, respectively. ORR were 22% in arm 1 (2 partial responses), 0% in arms 2, 3 and 4. Median PFS were 2.8, 2.1, 1.5 and 1.5 months, respectively. Median OS was not reached in arm 1 and 7.0, 2.7 and 3.8 months in arms 2, 3 and 4. Most common AEs were infusion-related reaction in 18/25 (72%; grade 1-2) during CBP501 infusion, and anemia in 2/33 (6%; both grade 3). SAEs occurred in 14/33 (42%); 13 SAEs were unrelated to CBP501. SAE acute renal failure (arm 3, after 1 cycle) was assessed as possibly related to CBP501 and definitely related to cisplatin.
It can be concluded that preliminary results indicate clinically meaningful improvement and tolerable safety of CBP501, cisplatin and nivolumab as 3rd line treatment for metastatic PDAC.
How to Easily View the Clinical Results Using Synapse Database?
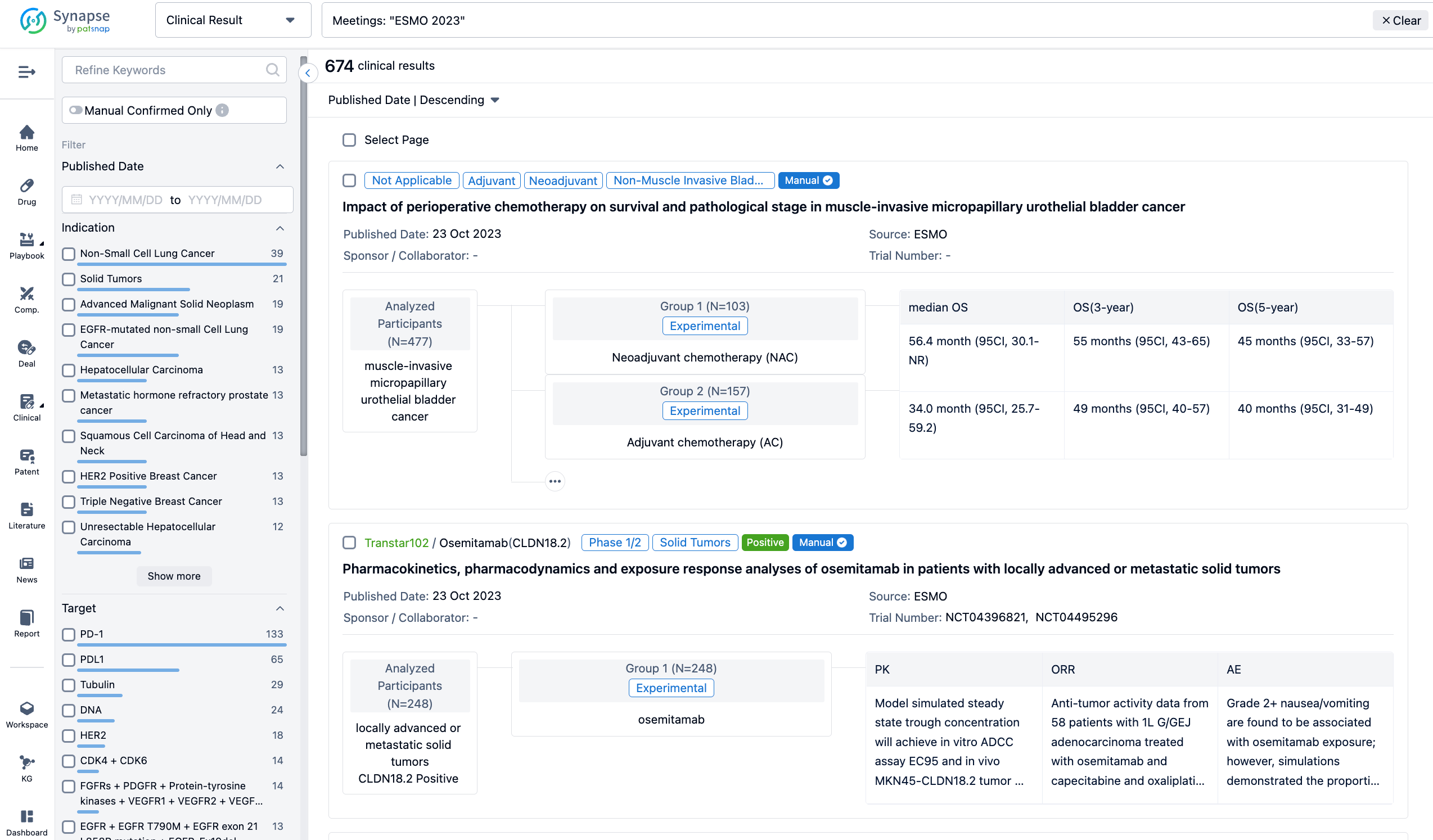
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
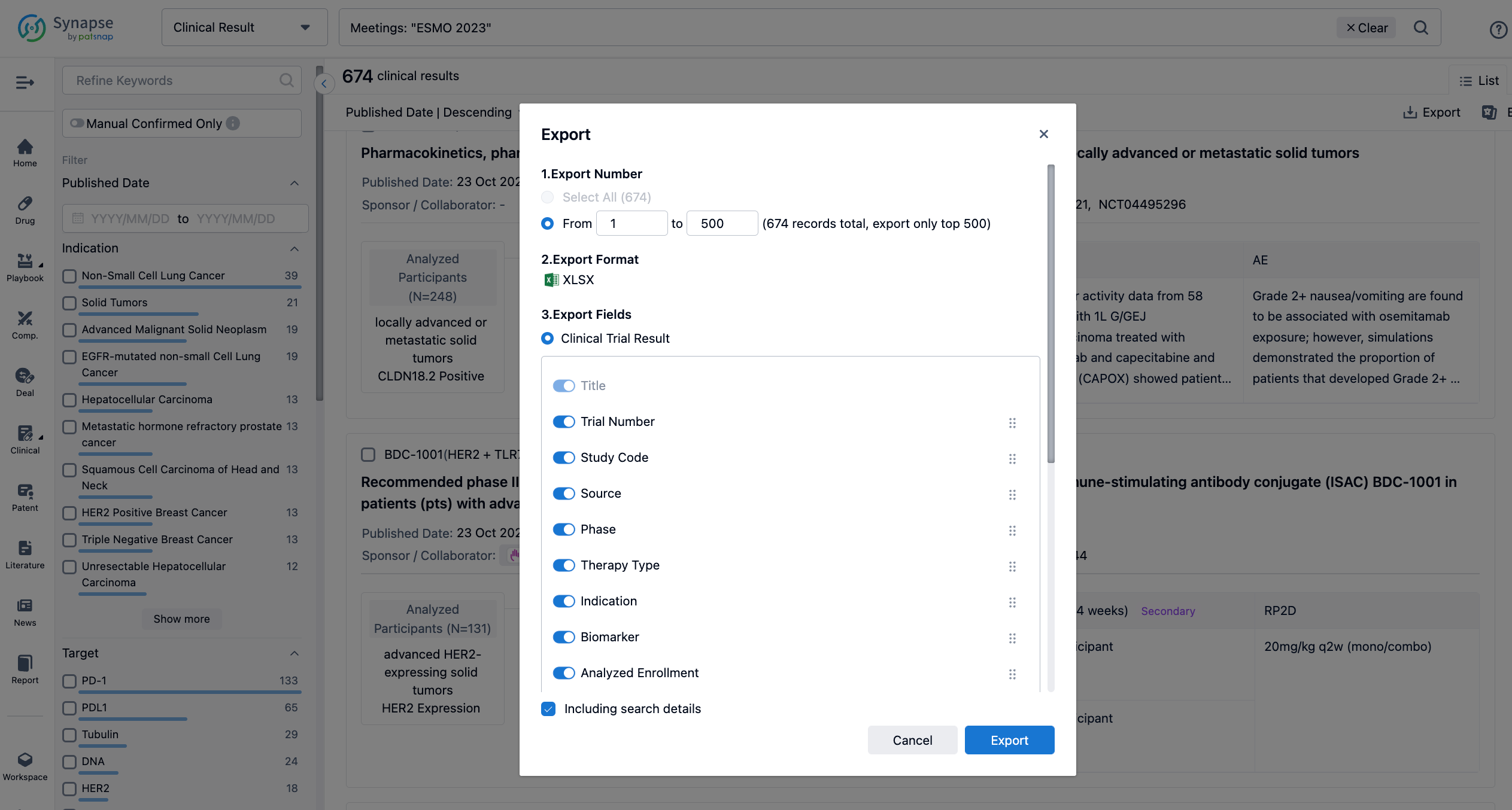
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
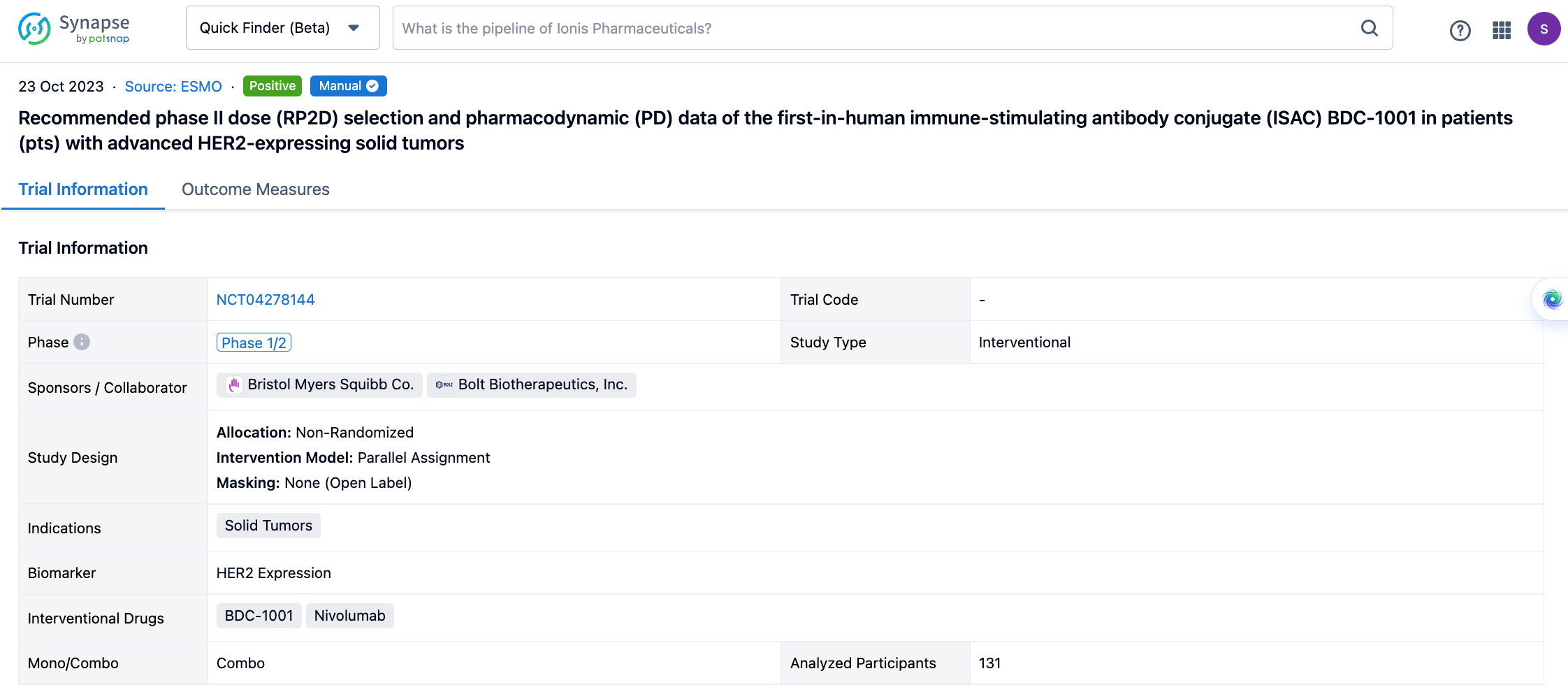
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!