Sarepta Therapeutics Gains Expanded FDA Approval for ELEVIDYS in Duchenne Muscular Dystrophy Patients Aged 4+
Sarepta Therapeutics, Inc., the leader in precision genetic medicine for rare diseases, announced U.S. Food and Drug Administration approval of an expansion to the labeled indication for ELEVIDYS (delandistrogene moxeparvovec-rokl) to include individuals with Duchenne muscular dystrophy with a confirmed mutation in the DMD gene who are at least 4 years of age. Confirming the functional benefits, the FDA granted traditional approval for ambulatory patients.
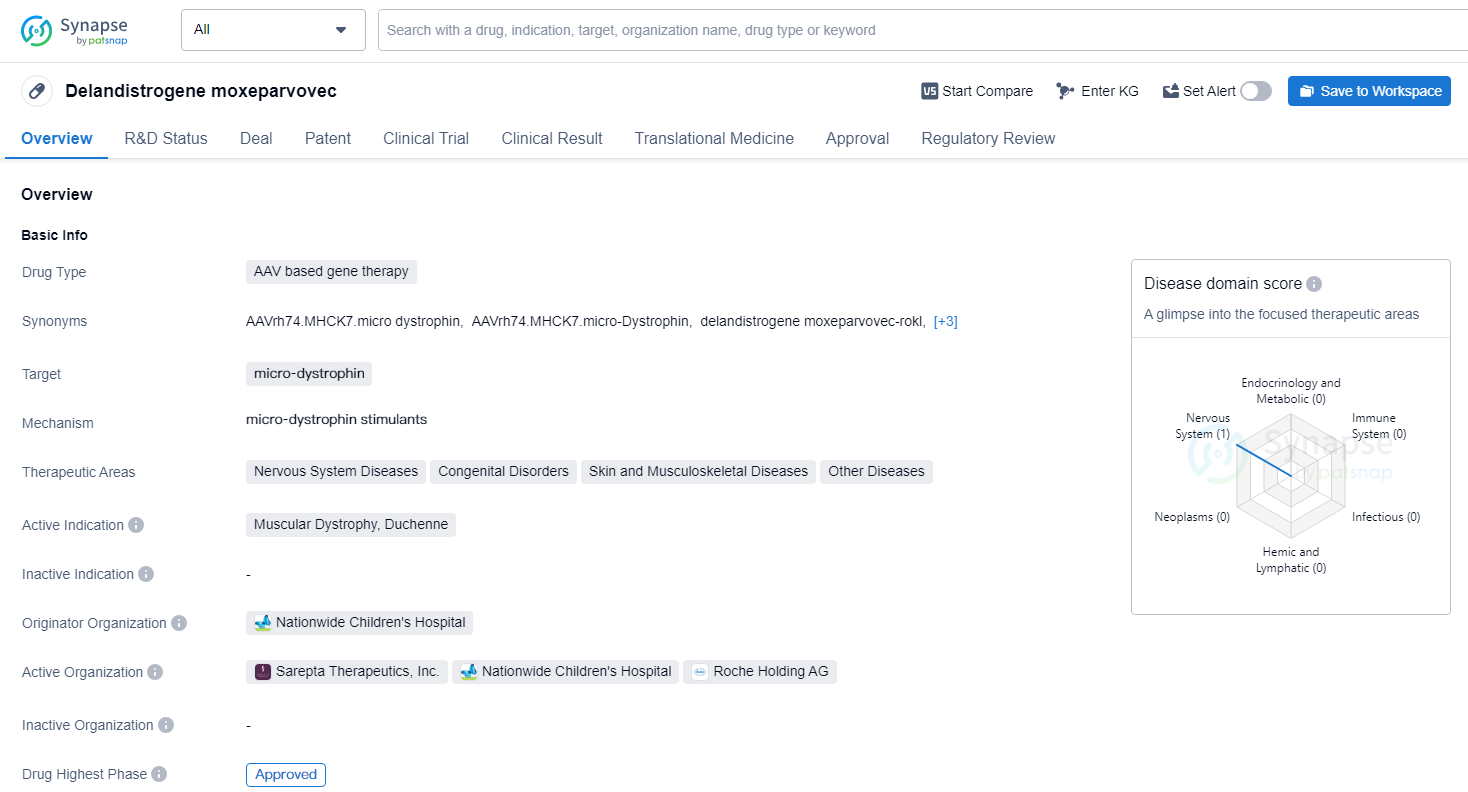
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The FDA granted accelerated approval for non-ambulatory patients. Continued approval for non-ambulatory Duchenne patients may be contingent upon verification of clinical benefit in a confirmatory trial. ELEVIDYS is contraindicated in patients with any deletion in exon 8 and/or exon 9 in the DMD gene.
“Representing many years of dedicated research, development, investment and creative energy, the expansion of the ELEVIDYS label to treat Duchenne patients aged 4 and above, regardless of ambulatory status, is a defining moment for the Duchenne community. Today also stands as a watershed occasion for the promise of gene therapy and a win for science,” said Doug Ingram, president and chief executive officer, Sarepta.
“At this pivotal moment, I want to give warm thanks to Drs. Jerry Mendell and Louise Rodino-Klapac for their dogged, 20-year pursuit of a gene therapy to treat this ruthless and life-robbing disease, to the FDA for following the scientific evidence to speed delivery of a therapy for a life-threatening rare disease to waiting patients, and to the many clinical investigators and courageous Duchenne families who have participated in the multiple studies that led to this important day.” Doug Ingram added.
Consistent with the accelerated approval pathway, Sarepta has committed to conduct and submit the results of a randomized, controlled trial to verify and confirm the clinical benefit of ELEVIDYS in patients with Duchenne muscular dystrophy who are non-ambulatory. ENVISION, a global, randomized, double-blind, placebo-controlled Phase 3 study of ELEVIDYS in non-ambulatory and older ambulatory individuals with Duchenne, is underway and intended to serve as this postmarketing requirement.
As part of a collaboration agreement signed in 2019, Sarepta is working with Roche to transform the future for the Duchenne community, enabling those living with the disease to maintain and protect their muscle function. Sarepta is responsible for regulatory approval and commercialization of ELEVIDYS in the U.S., as well as manufacturing. Roche is responsible for regulatory approvals and bringing ELEVIDYS to patients across the rest of the world.
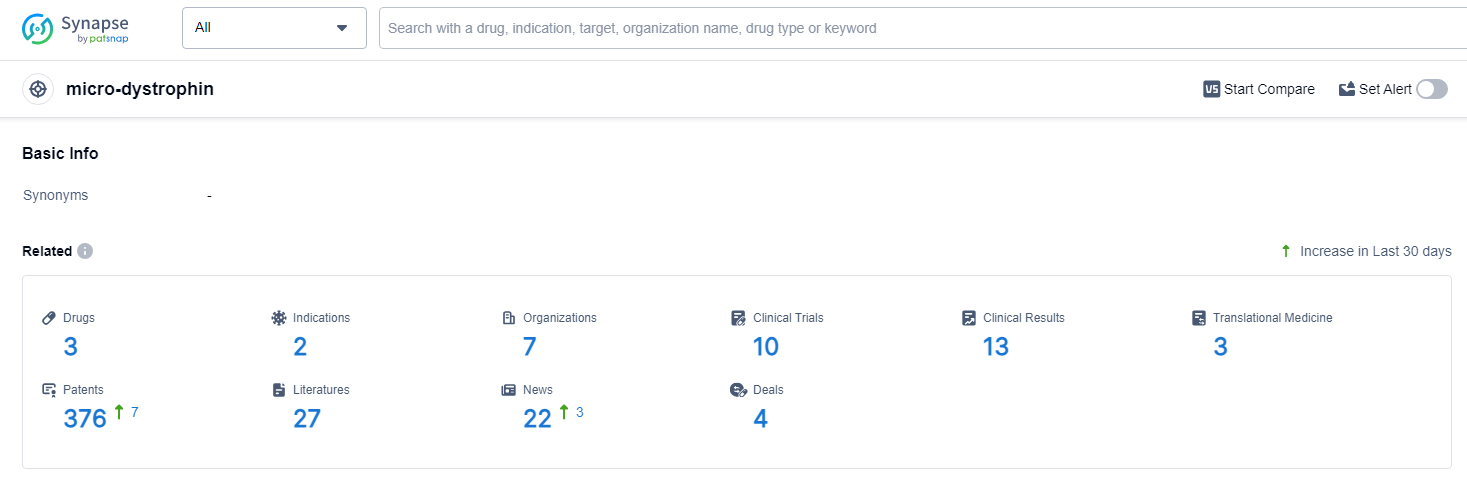
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 24, 2024, there are 3 investigational drugs for the micro-dystrophin target, including 2 indications, 7 R&D institutions involved, with related clinical trials reaching 10, and as many as 376 patents.
Delandistrogene moxeparvovec targets micro-dystrophin and is intended for the treatment of Muscular Dystrophy, Duchenne, as well as other nervous system diseases, congenital disorders, skin and musculoskeletal diseases, and other diseases. The approval of Delandistrogene moxeparvovec represents a significant advancement in the field of gene therapy and offers hope for patients with Duchenne muscular dystrophy and other related conditions.