The Latest Review Provides Detailed Analysis of CAR-NK and CAR-T Cell Therapies
In May 2024, the first girl ever cured by CAR-T cell immunotherapy, Emily, celebrated 12 years of cancer-free survival. Every year, Emily shares her cancer battle photos on social media. Now studying at the prestigious University of Pennsylvania, she has evolved beyond being just the first patient cured by CAR-T therapy to also being an advocate for cancer cures through cellular immunotherapy. She spreads solace in innovative medications, along with courage and confidence through the cancer-fighting process, globally.

Despite significant successes, CAR-T therapy faces challenges related to side effects, T cell exhaustion, and the hostile tumor microenvironment (TME). The production scaling process is currently both time-consuming and costly, making it difficult to extend CAR-T cell immunotherapy to a wider patient population.
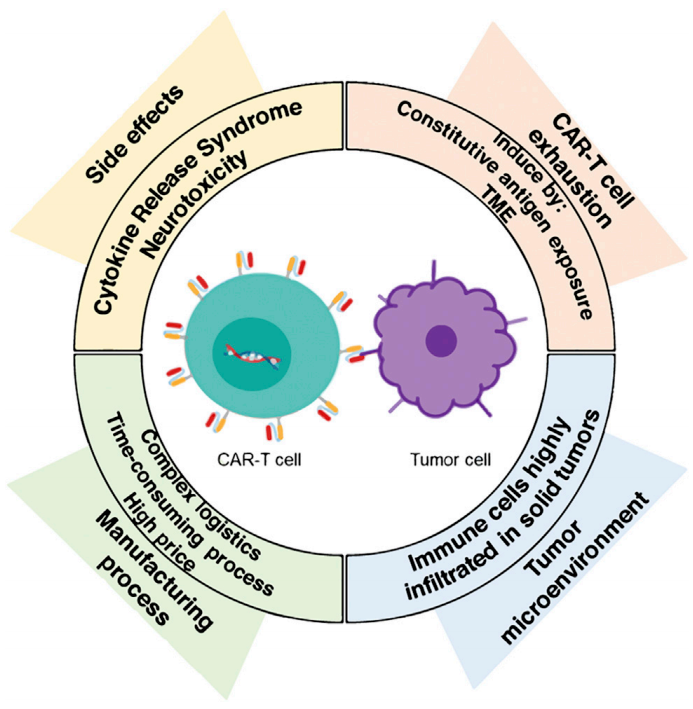
The improved version of CAR-T therapy, CAR-NK therapy, has recently attracted considerable attention from the academic and pharmaceutical sectors due to its increased tumor-specific targeting and cytotoxicity, cost-effectiveness and easier availability of NK cells, shorter large-scale production cycles, and enhanced efficacy in treating solid tumors.
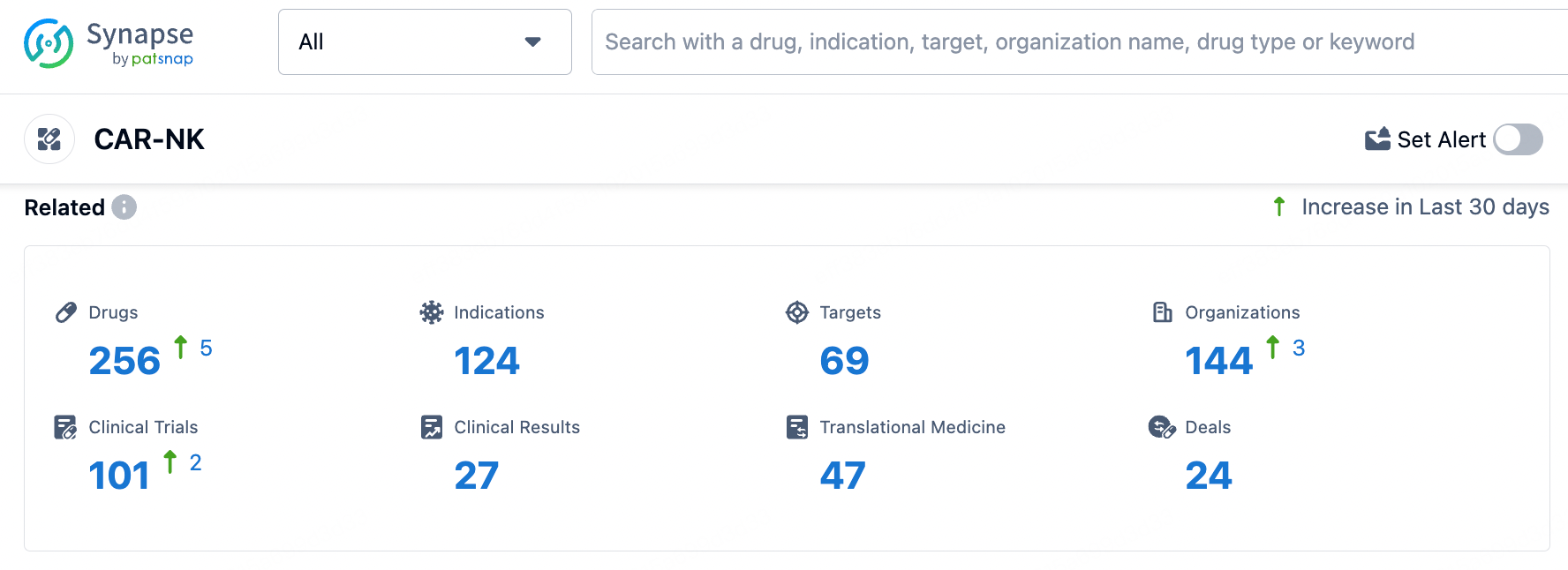
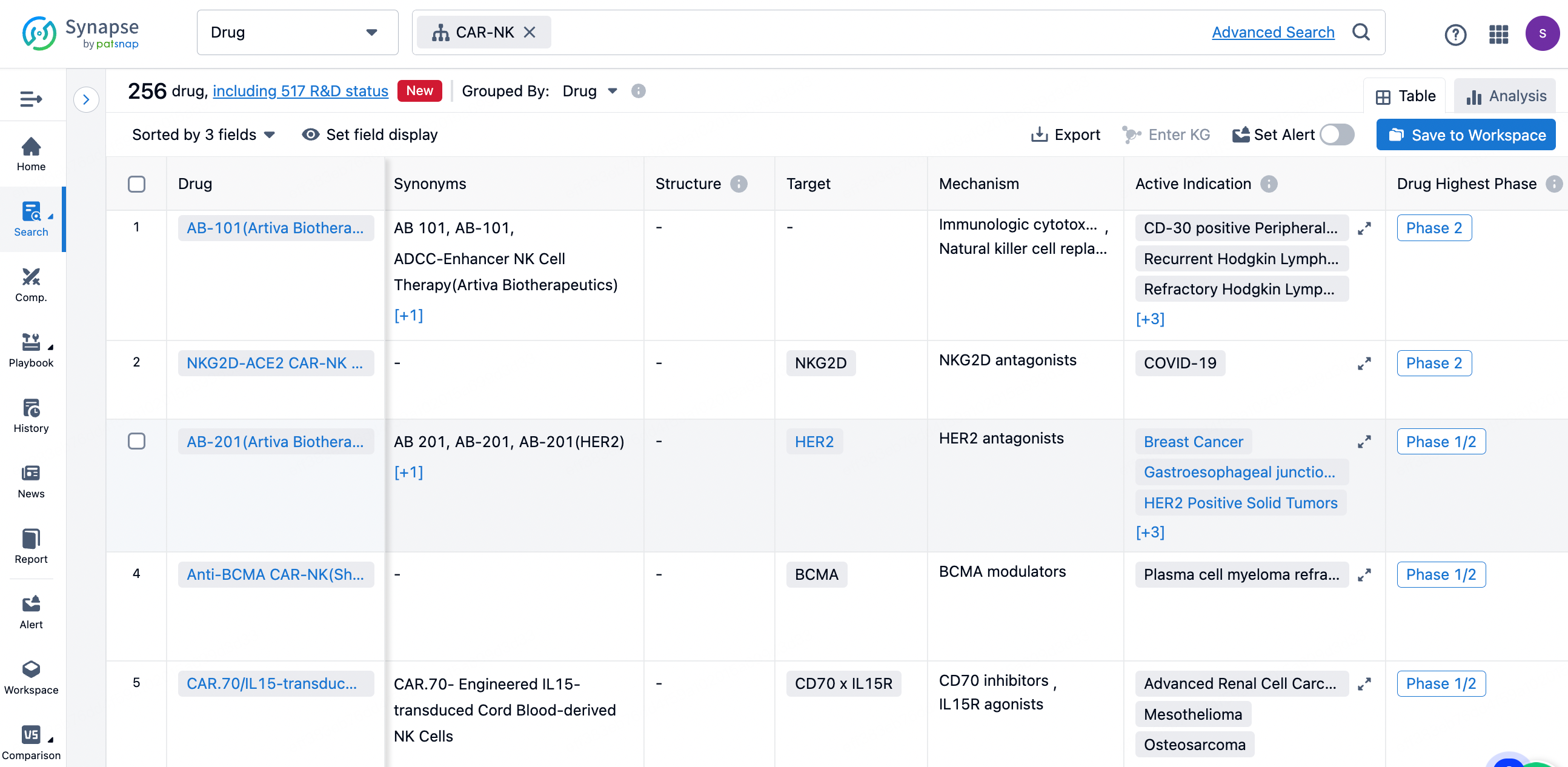
In the Synapse database, entering the keyword 'CAR-NK' retrieves a wide range of data including drugs, indications, targets, institutions, clinical trials, clinical outcomes, translational medicine, and drug deals.
This new type of immunocellular therapy technology, CAR-NK, has the following advantages:
·It is engineered from natural killer cells, resulting in lower toxic side effects and enhanced safety.
·It has a stronger ability to recognize and kill tumor cells, leading to more significant treatment effects.
·It facilitates more personalized treatments and has a wider range of applicability.
However, the CAR-NK technology also has some drawbacks, including:
·Current research and clinical applications are still in the early stages, requiring more time and resources for refinement and validation.
·Compared to CAR-T technology, research and development of CAR-NK are relatively lagging, resulting in significant market competition, although this also presents opportunities.
According to statistics from the Synapse database, as of June 25, 2024, there are a total of 256 CAR-NK therapies globally, originating from 144 institutions, targeting 69 different markers, and covering 124 indications, with 101 clinical trials being conducted.
The following sections will discuss the specific therapeutic advantages of CAR-NK mechanisms, as well as emerging opportunities in targets and indications.
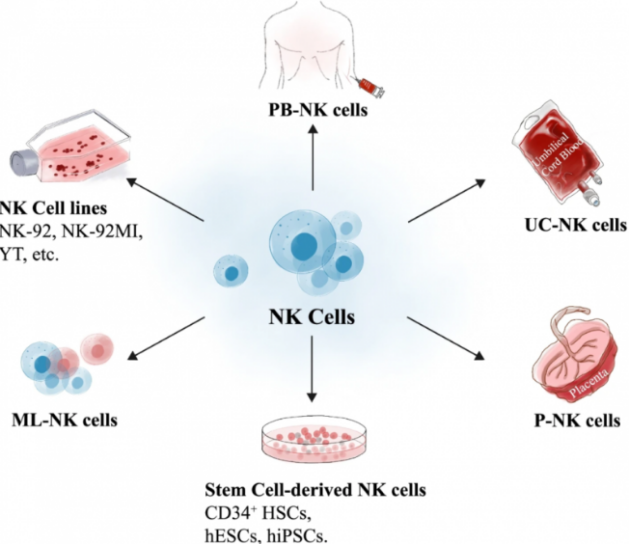
Natural Killer (NK) cells are a type of lymphocyte in the immune system that play a crucial role in defending the body against tumors and viral infections. Functionally similar to CD8+ cytotoxic T cells, NK cells kill target cells through similar cytotoxic mechanisms but lack somatic recombination and antigen-specific TCRs. Tumor cells with lower expression of Human Leukocyte Antigen (HLA) may be more susceptible to NK cell-mediated killing due to reduced inhibition mediated by KIRs.
As depicted above, NK cells can originate from various sources, such as peripheral blood mononuclear cells, umbilical cord blood, immortalized cell lines, hematopoietic stem and progenitor cells (HSPCs), and induced pluripotent stem cells (iPSCs).
Due to the phenotypic dysregulation of autologous NK cells in cancer patients, allogeneic NK cells often outperform autologous ones. NK cell lines, such as NK-92mi, have also been approved to be engineered with chimeric antigen receptors (CAR) to create CAR-NK therapeutic systems for clinical applications. This further enhances the sources and availability of NK cells.
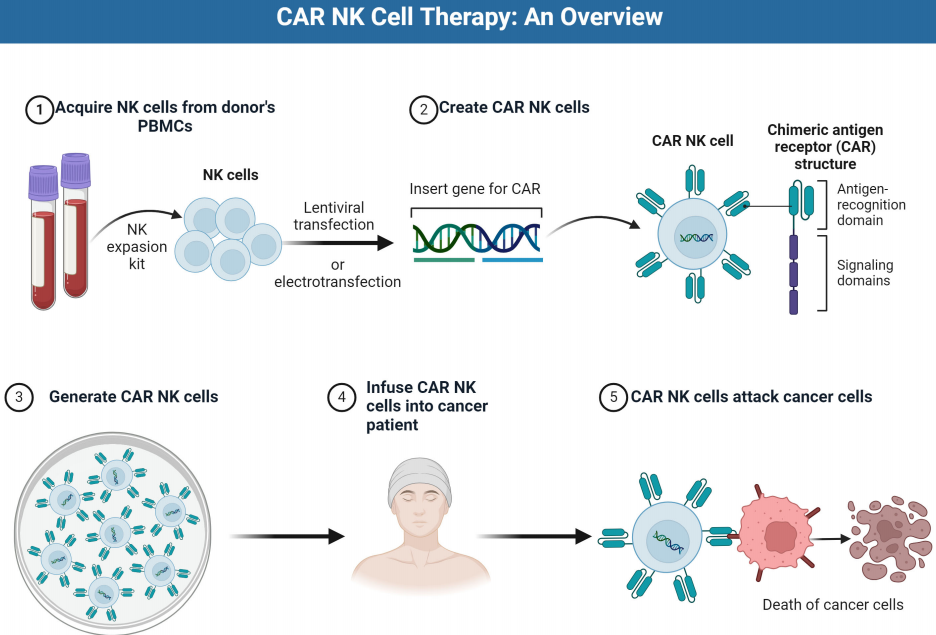
NK-92 cells, compared to NK cells derived from other sources, exhibit predictable proliferation kinetics, can be cultured in bioreactors, and are capable of generating billions of cells within weeks. Additionally, the NK cell line can be readily and efficiently transduced. There are already versions of NK-92 cells expressing chimeric antigen receptors (CARs) targeting various cancer cell surface receptors, such as CD19 (a B-cell receptor), human epidermal growth factor receptor 2 (HER2/ErbB2), and epidermal growth factor receptor (EGFR), with many of these modified NK-92 cells currently undergoing clinical trials for cancer treatment. CAR-NK cells have been engineered to recognize and attack specific antigens present on cancer cells. The upper figure illustrates the treatment process of CAR-NK cells using PBMCs as an example.
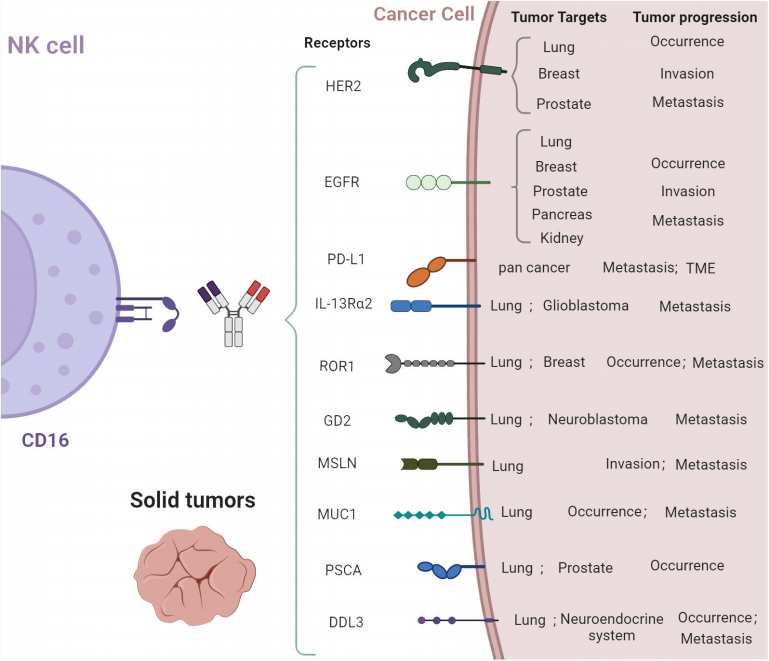
Many tumors may produce a variety of different genetic mutations, some of which may facilitate the spread of cancer cells to other parts of the body. For instance, mutations in the epidermal growth factor receptor (EGFR) have been identified as crucial drivers of metastasis, and safe and effective therapies have been developed to target mutated EGFR and inhibit cancer spread. These targets can also serve as the basis for designing the antigen-recognition domains of CAR-NK therapies. The targets listed in the lower figure, such as HER2, EGFR, PD-L1, ROR1, GD2, MSLN, etc., represent potential targets for CAR-NK cell therapies.
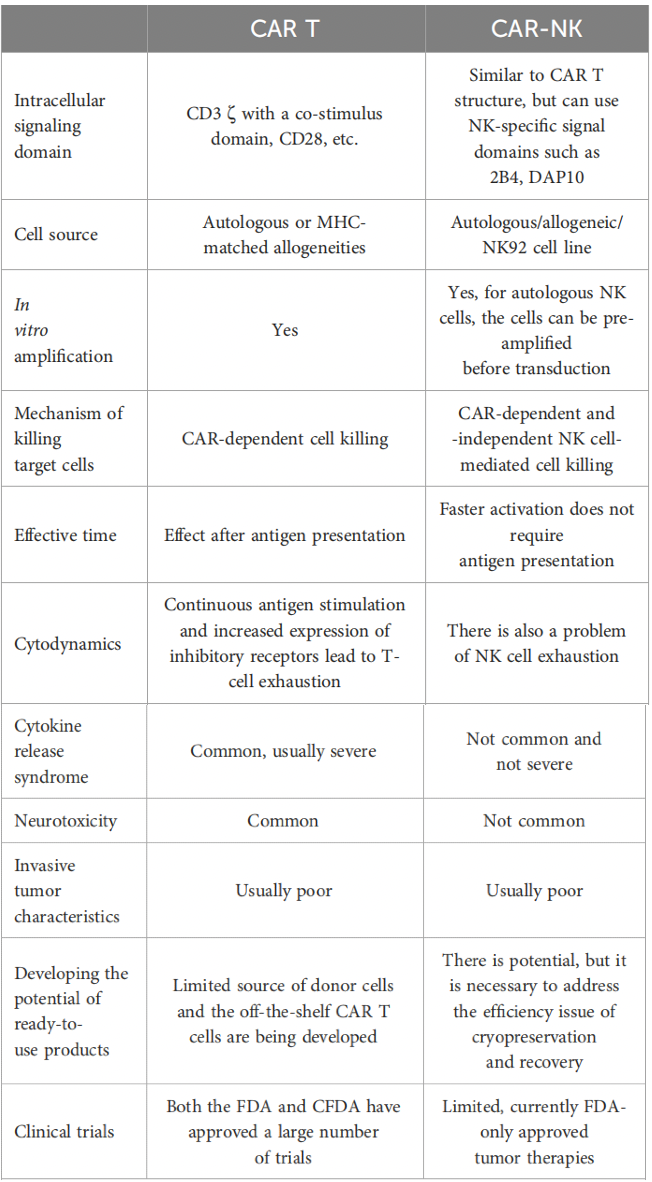
What are the similarities and differences between CAR-NK and CAR-T? Both utilize the human immune system to target and eliminate cancer cells, fundamentally transforming cancer treatment. Compared to CAR-T, one of the most notable advantages of CAR-NK therapy is its inherent high specificity in targeting cancer cells, while minimizing off-target effects. The following table compares the technologies of CAR-T and CAR-NK.
NK cells have a natural ability to distinguish between healthy and malignant cells, thereby reducing the risk of autoimmune reactions and collateral damage. Additionally, CAR-NK therapy has demonstrated promising efficacy in the treatment of solid tumors (such as lung cancer), which represents a significant challenge for other forms of immunotherapy. The inherent ability of NK cells to infiltrate solid tumors, combined with the specificity of CAR targeting, provides an effective treatment option for patients with solid tumors.
Unlike CAR-T therapy, CAR-NK therapy exhibits higher therapeutic safety. CAR-T cell therapy is associated with cytokine release syndrome (CRS), a potentially fatal immune reaction. CAR-NK cells show a lower tendency to induce CRS, making CAR-NK treatment a safer choice. This reduced risk enhances patient safety and allows for broader clinical applicability.
The sources of cells in cell therapy often become a limiting factor in clinical applications. CAR-T cells are typically autologous (from the patient), which limits their wider application. Currently, many companies are working to produce off-the-shelf CAR-T cell products, such as employing induced pluripotent stem cells (iPSCs) for differentiation into T cells, which are under development. Unlike T cells, NK cells can be obtained from allogeneic sources (donors), making them easily available for off-the-shelf use. This allogenic capability significantly reduces the time and cost associated with CAR-NK cell therapies, making them more accessible to a broader patient population. The primary sources of NK cells include peripheral blood, umbilical cord blood, NK cell lines, and induced pluripotent stem cells (iPSCs). Peripheral blood is the most traditional source of NK cells for therapeutic purposes. NK cells derived from peripheral blood are readily available and can be collected from patients (autologously) or donors (allogeneically). Peripheral blood collection is relatively simple, and autologous use minimizes the risk of immune rejection.
In addition to peripheral blood, umbilical cord blood (UCB) is another explored source of NK cells due to its unique properties. NK cells derived from UCB exhibit a higher degree of immaturity, which leads to better expansion and longevity after infusion. When used allogeneically, they also carry a lower risk of causing graft-versus-host disease (GvHD).
Beyond these naturally derived NK cells, NK cells obtained through cellular engineering have also been demonstrated to be capable of being equipped with CAR structures for constructing CAR-NK therapies. iPSCs represent a cutting-edge source for NK cells, capable of differentiating into any cell type, including NK cells. iPSCs provide an inexhaustible source of NK cells that can be genetically engineered to enhance their anti-cancer properties. Although the differentiation process is complex and costly, the long-term safety of iPSC-derived NK cells remains to be fully determined. Certain human NK cell lines, such as NK-92, have been genetically modified to express therapeutic uses. NK cell lines offer a consistent and limitless source of NK cells that can be easily engineered and expanded in vitro. The use of cell lines facilitates the expansion and packaging processes, while also reducing the batch effects and instability associated with engineered cells.
Conclusion
CAR-NK cell therapy represents a promising approach in cancer treatment. Compared to traditional therapies, it offers potential advantages including reduced risks of CRS (Cytokine Release Syndrome) and GvHD (Graft-versus-Host Disease), along with the capacity to target and kill cancer cells through innate immune mechanisms. Addressing challenges related to TME (Tumor Microenvironment) suppression, CAR-NK cell persistence, antigen escape, and scalability is crucial for the successful development and clinical implementation of CAR-NK cell therapy.
Leveraging advances in genetic engineering, immunology, and manufacturing technologies, CAR-NK cell therapy holds significant potential to enhance efficacy, safety, and accessibility, potentially offering new hope for cancer patients.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference
1.Xiao song, et al;Advances in CAR-NK cell therapy for lung cancer: is it a better choice in the future? DOI 10.3389/fonc.2024.1390006.
2.Zhang et al, (2022). CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomarker Research,https:/doi.org/10.1186/s40364-022-00364-6.