Chugai collaborates with Helsinn to promote the antiemetic drug AKYNZEO
On June 12, Swiss pharmaceutical company Helsinn Healthcare announced the renewal of its distribution and licensing agreement with Chugai Pharma Europe, the European subsidiary of Japanese pharmaceutical company Chugai, to promote AKYNZEO® (a combination drug containing netupitant and palonosetron) in the UK and Ireland.

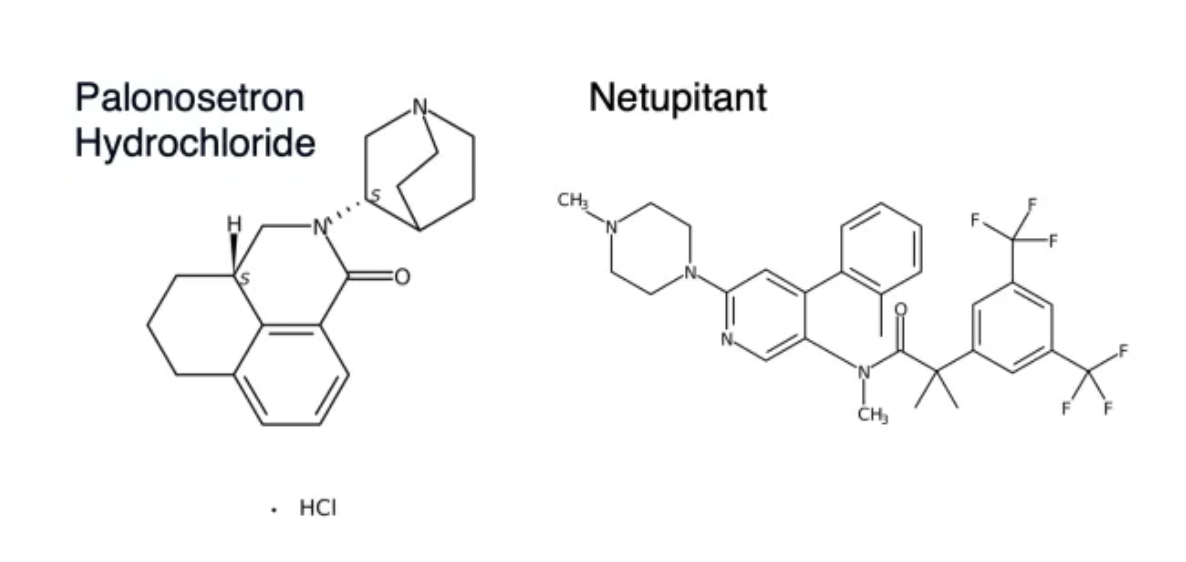
It is reported that AKYNZEO® was first approved in the United States by the FDA in 2014 as a medication used to treat chemotherapy-induced nausea and vomiting. It consists of two active ingredients: netupitant and palonosetron. Netupitant is a neurokinin NK1 receptor antagonist that selectively blocks the activation of NK1 receptors, reducing delayed nausea and vomiting caused by chemotherapy. Palonosetron is a serotonin 5-HT3 receptor antagonist that blocks the activation of 5-HT3 receptors, reducing acute nausea and vomiting induced by chemotherapy.
The indication for AKYNZEO® primarily involves the prevention of acute and delayed nausea and vomiting associated with highly or moderately emetogenic chemotherapy in adults. It is typically used in conjunction with dexamethasone to enhance the preventive effect. The pharmaceutical forms of the drug include capsules and injections, with each capsule containing 300 mg of netupitant and 0.5 mg of palonosetron. The recommended dosage and administration of AKYNZEO® is to take one capsule orally at least one hour before the start of chemotherapy, and only once per chemotherapy cycle. However, use should be avoided or administered with caution under physician guidance in patients with severe hepatic impairment, severe renal impairment or end-stage renal disease, and pregnant or breastfeeding women.
Common adverse reactions of AKYNZEO® include headache, constipation, fatigue, and stomach discomfort. AKYNZEO® is an effective supportive medication for chemotherapy, significantly improving the quality of life during chemotherapy by alleviating symptoms like nausea and vomiting. When using this medication, patients should follow the guidance and recommendations of their physician to ensure safe and effective use.
Nausea and vomiting are common physical discomfort symptoms that can be caused by a variety of reasons. Nausea is a sensation of unease in the stomach that may be accompanied by an urge to vomit, while vomiting involves the forceful expulsion of stomach contents through the mouth. Nausea and vomiting can be symptoms of many different health conditions, including early pregnancy, concussion, cancer chemotherapy, and gastroenteritis.
Both adults and children can experience these symptoms, and methods to alleviate nausea vary from person to person. However, some common home remedies include drinking clear liquids or chilled beverages, eating bland foods such as saltine crackers or white bread, avoiding greasy or sweet foods, eating slowly, having smaller, more frequent meals, not mixing hot and cold foods, sipping beverages slowly, avoiding activity after eating, not brushing teeth immediately after meals, and choosing foods that are tolerable to ensure a balanced diet.
Chemotherapy-induced nausea and vomiting (CINV) are common side effects during chemotherapy processes, significantly affecting the quality of life for cancer patients undergoing treatment and are among the most feared adverse events associated with chemotherapy. CINV not only impacts the patient's day-to-day life but can also cause psychological stress, sometimes even leading patients to delay or refuse necessary chemotherapy treatments.
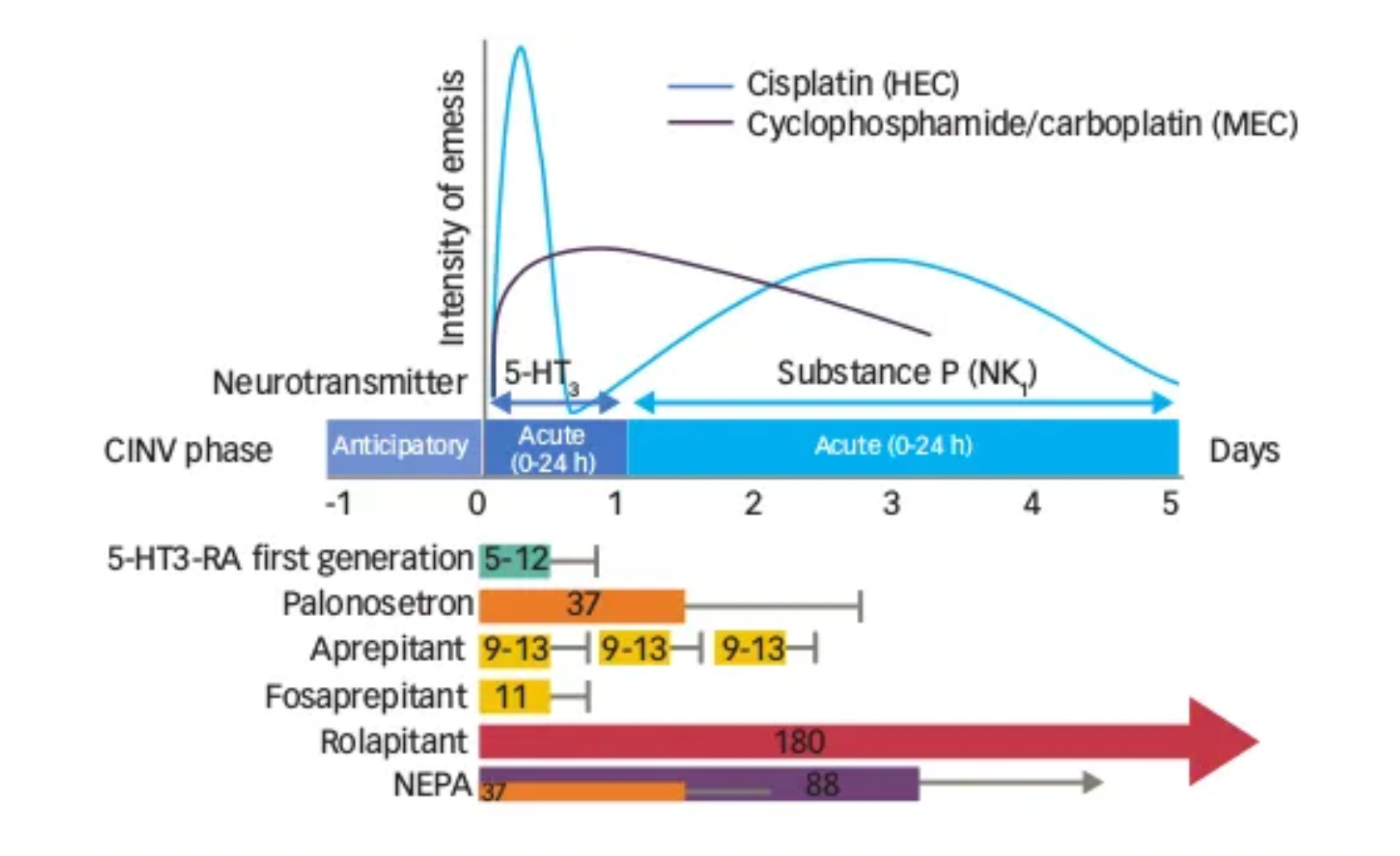
The occurrence of CINV (Chemotherapy-induced Nausea and Vomiting) presents two peak periods: an acute phase within 1-2 hours post-chemotherapy, known as Acute CINV, and another peak occurring between 48-72 hours after chemotherapy, which can last several days, known as Delayed CINV. The incidence of Delayed CINV is approximately twice that of Acute CINV. However, during the delayed phase of chemotherapy, patients often no longer have direct contact with the oncology medical team, posing challenges for clinical management. Moreover, most antiemetic treatments are more effective against Acute CINV than Delayed CINV.
The pathogenesis of CINV involves both the central and peripheral nervous systems. Chemotherapy drugs lead to the release of various neurotransmitters in the gastrointestinal tract, cerebral cortex, thalamus, vestibular areas, and the area postrema, which includes dopamine, endorphins, serotonin, and neurokinin, with substance P as a receptor ligand. 5-HT3 receptor antagonists (5-HT3 RAs) have shown effectiveness in controlling acute CINV but have limited efficacy in Delayed CINV. In recent years, the introduction of neurokinin-1 (NK-1) receptor antagonists has represented a relevant advancement in preventing CINV associated with highly and moderately emetogenic chemotherapy, particularly in the delayed phase.
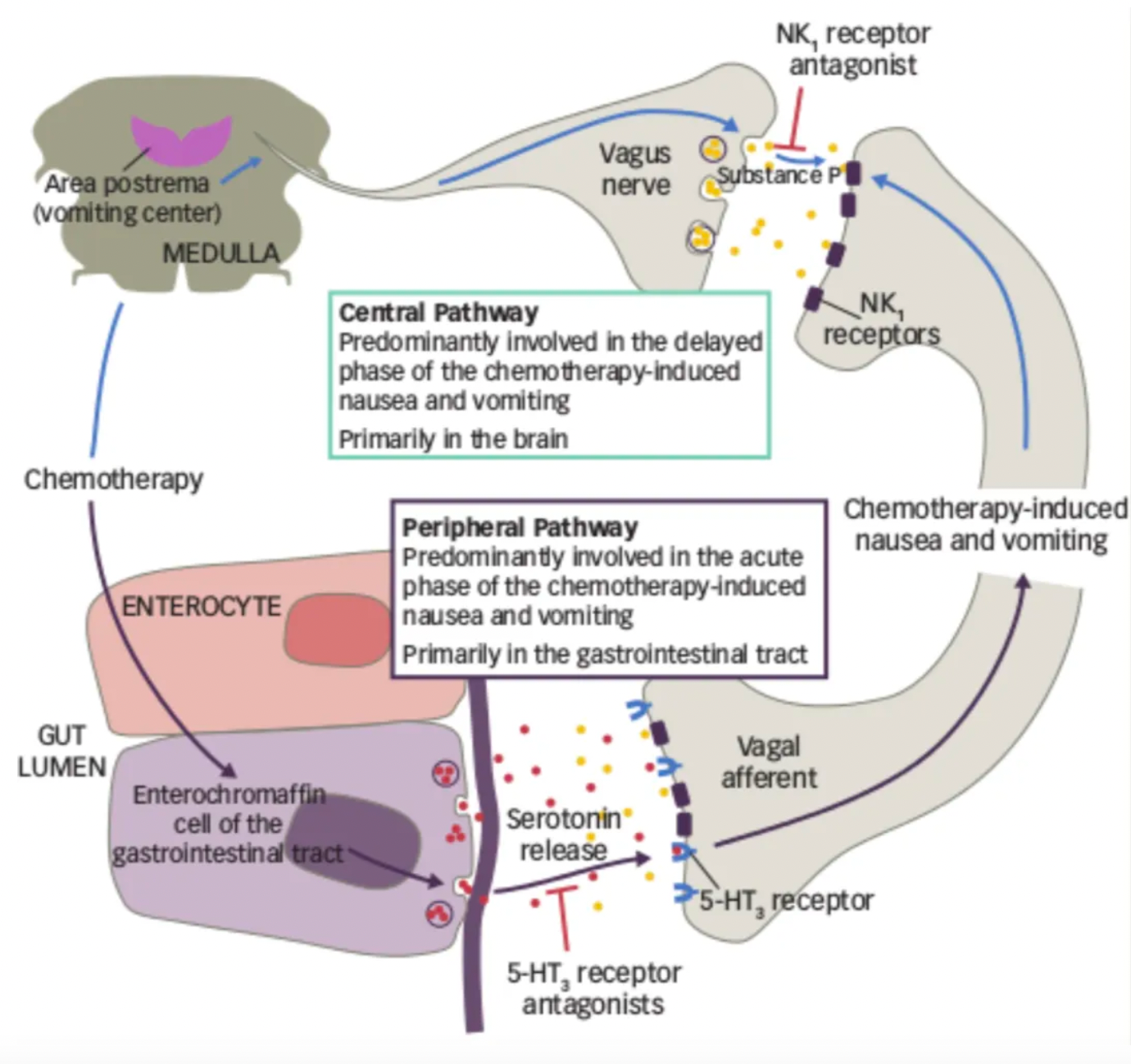
Anti-emetic drugs targeting the NK1R receptor include:
·Aprepitant, the first NK1R receptor antagonist approved globally, was developed and clinically indicated by Merck. Aprepitant can be used in combination with other anti-emetics to prevent acute and delayed nausea and vomiting associated with the initial and repeat courses of high emetogenic cancer chemotherapy.
·Fosaprepitant (L-758,298, MK-0517) is the second globally marketed NK1R receptor antagonist developed by Merck, with the initial indication still being chemotherapy-induced nausea and vomiting.
·Rolapitant (SCH-619734) is a highly selective NK1R receptor antagonist originally developed by Schering Plough Corporation.
·The mentioned combination of Netupitant/Palonosetron consists of the NK1R receptor antagonist Netupitant and the 5-HT3R antagonist Palonosetron. Developed by Helsinn Healthcare of Switzerland, it is indicated primarily for nausea and vomiting caused by chemotherapy.
In the targeted antiemetic drugs treating GPCR-related targets, besides NK1R receptors, agents that interact with receptors like D2R, CB, and 5-HT, also represent significant market opportunities.
As a relatively niche therapeutic avenue, currently, there are few GPCR-targeting antiemetic projects with marketing applications and clinical investigations. Prominent drugs include HR-20013, dronabinol hemisuccinate, and relamorelin. As reported, in December 2023, the CDE official site publicized that Suncadia Medicine, a subsidiary of Hengrui Pharmaceuticals, submitted an application for market approval of the injectable HR20013, which was accepted. HR20013 is a combination of NK-1RA and 5-HT3RA intended to be developed for the prevention of post-chemotherapy nausea and vomiting, with HR-20013 being an active component. Once administered, HRS5580 is rapidly converted into Rolapitant through non-CYP450 enzyme systems like thiol methyltransferase.
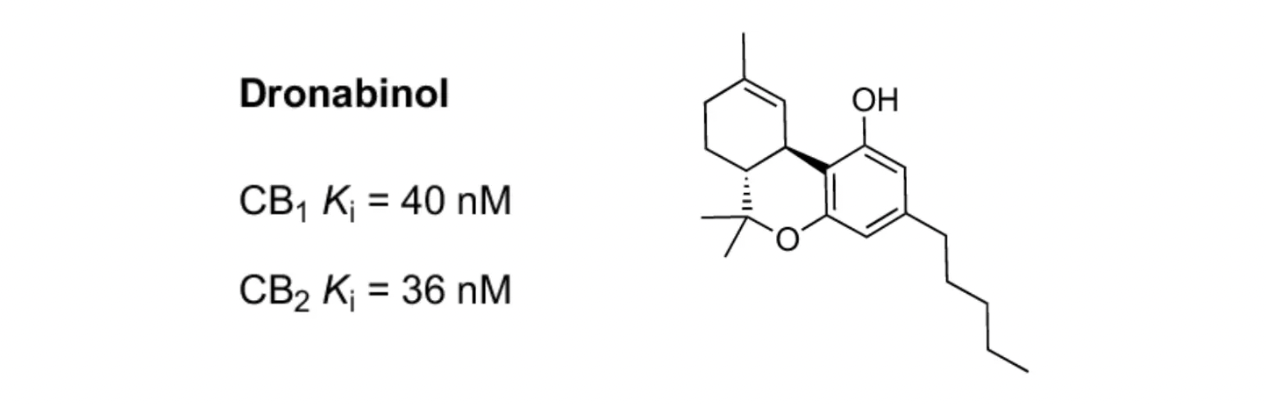
Dronabinol is another product developed by INSYS Therapeutics as a non-selective CB receptor agonist. It is also used to treat nausea and vomiting induced by chemotherapy, especially when other antiemetic drugs have not been effective. Dronabinol functions by activating cannabinoid receptors in the brain, helping to reduce the sensation of nausea and decreasing the frequency of vomiting. It is typically available in the form of oral capsules, taken before chemotherapy to prevent nausea and vomiting. Although Dronabinol can effectively alleviate nausea and vomiting caused by chemotherapy, it may also have some side effects, including dizziness, dry mouth, fatigue, and confusion. Before using Dronabinol or any other medication, patients should discuss the potential benefits and risks with their healthcare professionals.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!