Scemblix® by Novartis shows superior efficacy in a Phase III trial, significantly increasing molecular remission rates for new chronic myeloid leukemia cases
Novartis has shared encouraging findings from its initial evaluation of the ASC4FIRST study. This landmark Phase III trial is significant as it is the initial randomized and direct comparative study within a Phase III context to assess the effectiveness of Scemblix® (asciminib) against a selection of tyrosine kinase inhibitors (TKIs) chosen by the investigating researchers. This study concentrated on patients who have been recently diagnosed with chronic myeloid leukemia (CML) characterized by the presence of the Philadelphia chromosome and who are in the chronic phase of the disease. ASC4FIRST distinguishes itself by being the inaugural study to pit a CML therapeutic option, in this instance Scemblix® (asciminib), against the established first- and second-generation TKIs acknowledged as the standard treatment protocol.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The research achieved its two main goals by establishing a significant superiority in the major molecular response rate of Scemblix when compared to TKIs chosen by the researchers and when measured against imatinib, confirming that the outcomes were both clinically meaningful and statistically significant for the targets assessed.
In the safety and tolerability assessment, Scemblix presented fewer side effects and a decrease in the number of patients stopping treatment compared to standard-of-care TKIs chosen by the researchers. No additional safety concerns were identified during the ASC4FIRST study, reinforcing Scemblix's already known safety profile.
"The outcomes are highly promising, especially since a considerable segment of patients with a fresh diagnosis of chronic myeloid leukemia, or CML, struggle to meet their therapeutic objectives," mentioned Prof. Tim Hughes, MD, from the South Australian Health & Medical Research Institute. "There's a clear demand for more acceptable treatment alternatives in the first-line treatment of CML, which can improve patients' quality of life without compromising their treatment regimes."
"Scemblix offers a promising option for those just diagnosed with CML, aiming to help them reach their therapy targets while maintaining their day-to-day activities," stated Shreeram Aradhye, M.D., Novartis' President of Development and Chief Medical Officer. "Considering that patients may require TKI treatment for extended periods due to the chronic nature of CML, it's essential to have treatment choices that are both highly effective and have good tolerability to support long-term compliance."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
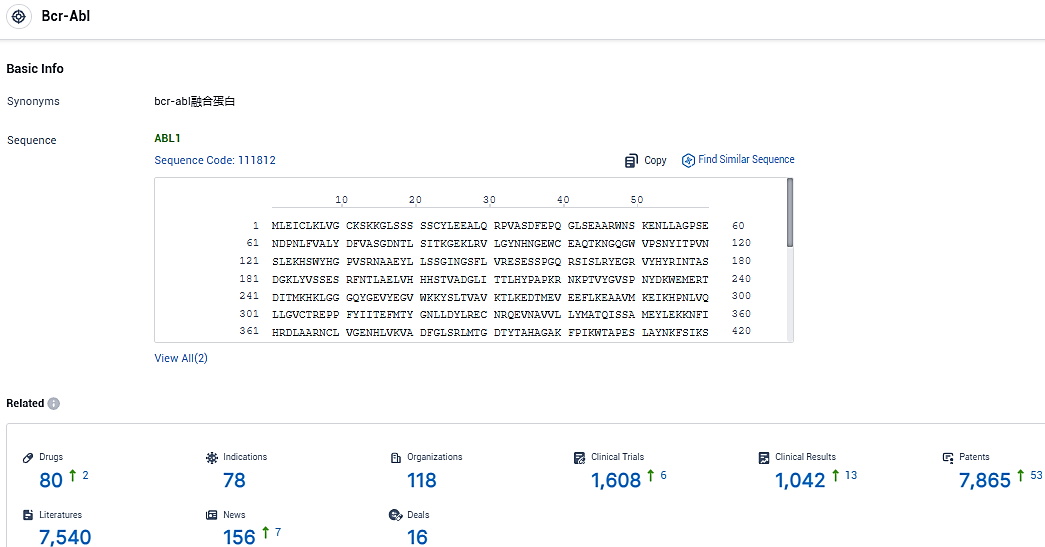
According to the data provided by the Synapse Database, As of January 12, 2024, there are 80 investigational drugs for the Bcr-Abl target, including 78 indications, 118 R&D institutions involved, with related clinical trials reaching 1608, and as many as 7865 patents.
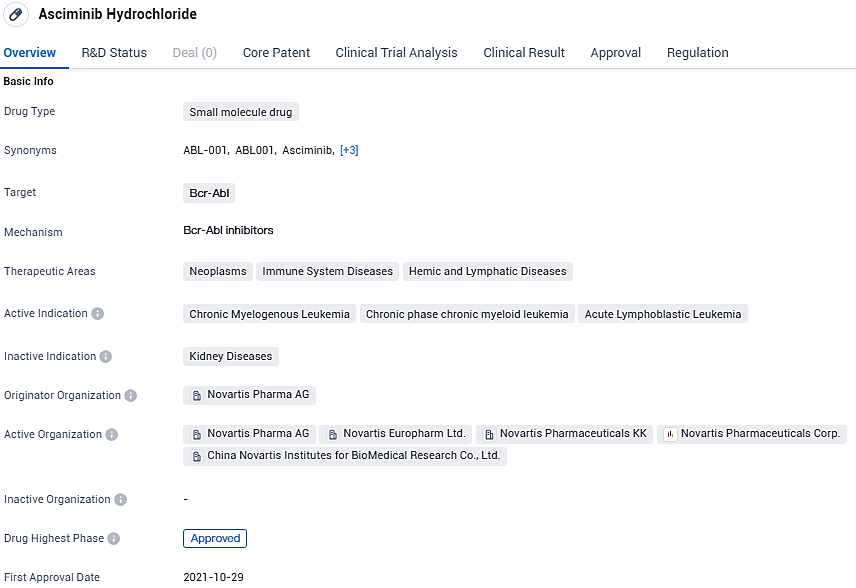
Asciminib targets Bcr-Abl and is primarily used in the treatment of neoplasms, immune system diseases, and hemic and lymphatic diseases. The drug has been approved globally and received its first approval in the United States in October 2021. It is currently in Phase 3 of development in China. Asciminib has received regulatory designations such as Priority Review, Fast Track, Orphan Drug, and Breakthrough Therapy, indicating its potential to address critical medical needs.