Merck Announces Phase 3 ZENITH Study Success for WINREVAIR™ (Sotatercept-csrk)
Merck (NYSE: MRK), referred to as MSD outside of the U.S. and Canada, announced that it achieved encouraging topline findings from the Phase 3 ZENITH trial, which assessed WINREVAIR (sotatercept-csrk) in adults diagnosed with pulmonary arterial hypertension (PAH, WHO* Group 1) who are classified as functional class (FC) III or IV and are at high mortality risk. The ZENITH trial successfully reached its primary goal of measuring the time to the first morbidity or mortality event, which includes all-cause death, lung transplants, or hospitalizations due to worsening PAH lasting 24 hours or more. In this trial, WINREVAIR showed a statistically significant and clinically relevant decrease in the risk of such morbidity or mortality events compared to the placebo, when administered alongside existing PAH treatments. Given the robust nature of these findings, an independent data monitoring committee has advised that the ZENITH study be halted prematurely, allowing all participants access to WINREVAIR via the SOTERIA open-label extension study. An initial review indicated that adverse events and serious adverse events occurred at similar rates in both treatment groups.
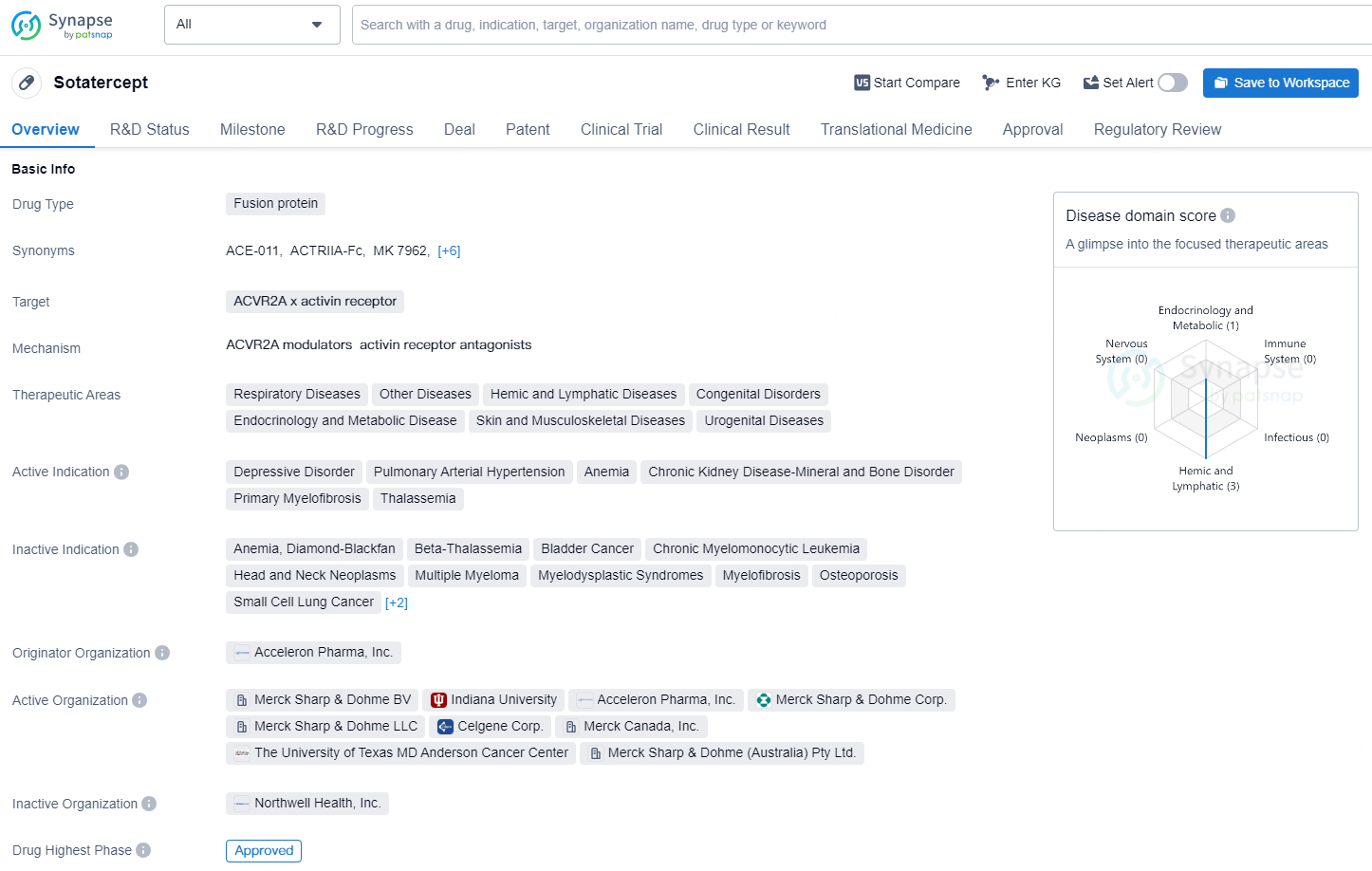
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
“PAH represents a severe, progressive condition characterized by significant morbidity and mortality rates,” stated Dr. Eliav Barr, Merck Research Laboratories' senior vice president and head of global clinical development, as well as chief medical officer. “Given the primary endpoint's demonstration of remarkable efficacy, all participants in the ZENITH study will have the chance to access WINREVAIR. These results are notable and set a high standard for future research involving potential treatments for PAH, highlighting WINREVAIR's ability to fundamentally change how PAH is managed.”
“The ZENITH trial was intended to determine if incorporating WINREVAIR, which acts as an activin signaling inhibitor, could lower the chances of mortality, lung transplants, or hospital admissions related to PAH for those with severe PAH,” explained Dr. Vallerie McLaughlin, the Kim A Eagle MD Endowed Professor of Cardiovascular Medicine and Director of the Pulmonary Hypertension Program at the University of Michigan in Ann Arbor. “This study marks the first instance in PAH research where the interim analysis prompted an early termination due to significant efficacy findings. WINREVAIR has injected a wave of hope into the field, and we express our gratitude to both the investigators and the participants for their contributions to this crucial study.”
WINREVAIR has received approval in the United States and 36 other countries, following results from the Phase 3 STELLAR trial. Most recently in November, WINREVAIR's approval was submitted in Japan, based on findings from the STELLAR trial alongside data from an open-label Phase 3 study involving Japanese patients.
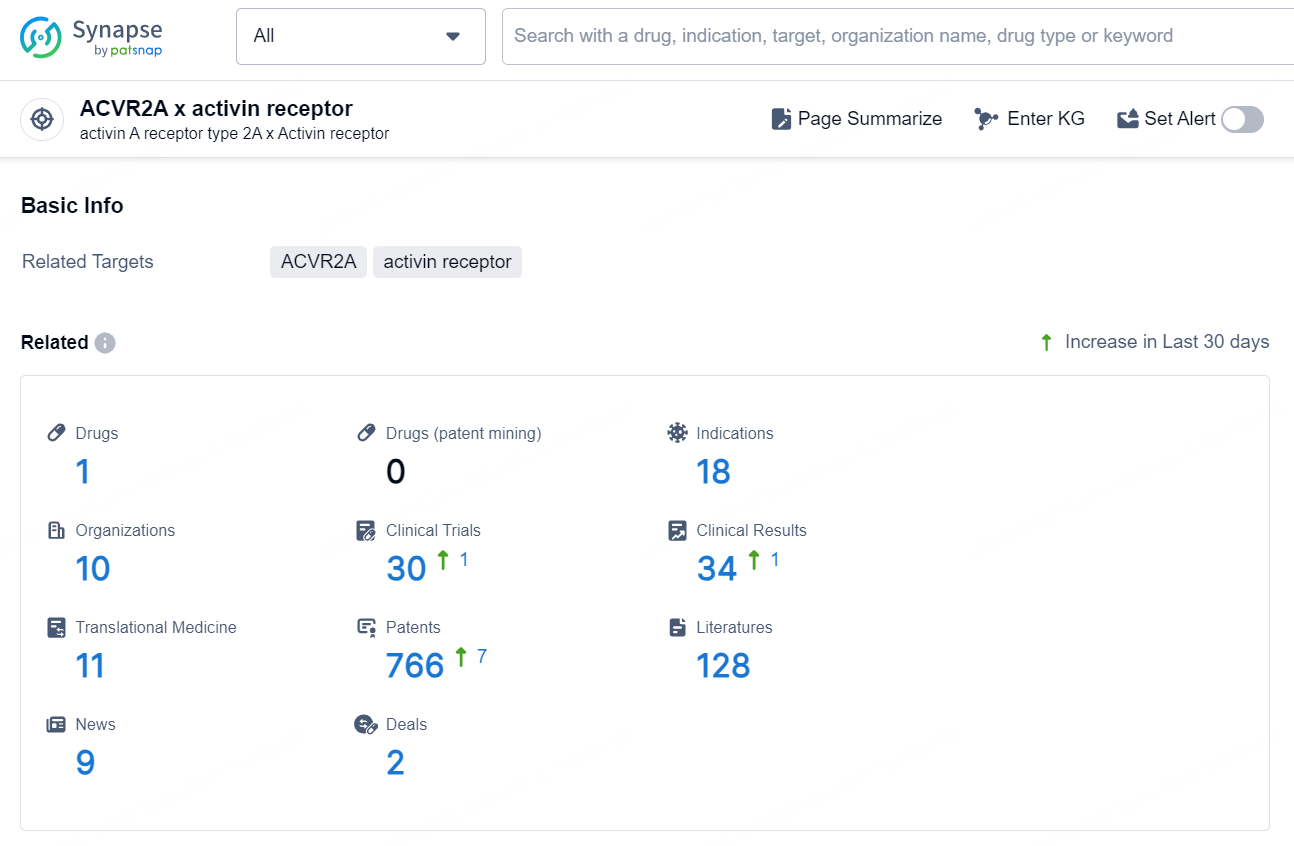
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of December 2, 2024, there are 1 investigational drug for the ACVR2A x activin receptor target, including 18 indications, 10 R&D institutions involved, with related clinical trials reaching 30, and as many as 766 patents.
Sotatercept is a fusion protein drug developed by Acceleron Pharma, Inc. The drug targets the ACVR2A x activin receptor and is indicated for a variety of therapeutic areas including respiratory diseases, hemic and lymphatic diseases, congenital disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, and urogenital diseases.