Seladelpar from CymaBay Reaches Significant Statistical Levels for primary and key Secondary endpoints in the RESPONSE Phase 3 Trial
CymaBay Therapeutics, Inc., a biotech company devoted to developing novel treatments for liver and other chronic diseases, announced encouraging primary results from its decisive Phase 3 RESPONSE trial. This research investigated the safety and performance of seladelpar, a strong selective oral delpar or PPARδ agonist under development for therapy of adult patients suffering from primary biliary cholangitis. The registration trial were successfully met primary and all key secondary endpoints, leading the way for regulatory dialogues and submissions for official authorization with FDA, MHRA, and EMA.
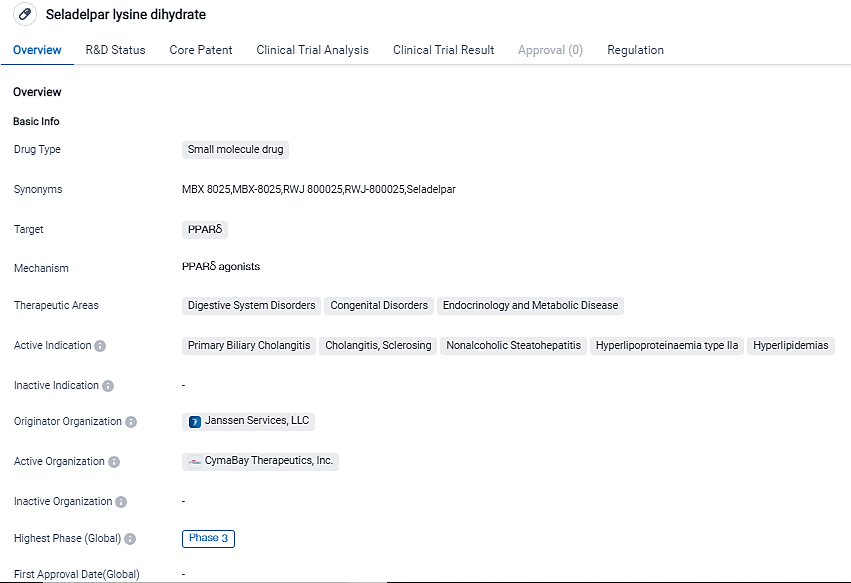
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
"The primary results gathered from the RESPONSE clinical trial are encouraging, they indicate the possibilities of a new, effective and safe therapeutic approach that not only delivers enhanced total improvements in liver evaluations, but also normalizes these evaluations for a considerable amount of patients.
Moreover, the outcomes confirm that seladelpar reduced itchiness, an especially complex symptom that consistently hampers many PBC patient's quality of life," said Gideon Hirschfield, M.D., Lily and Terry Horner Chair in Autoimmune Liver Disease Research, Toronto Centre for Liver Disease.
Sujal Shah, the President and CEO of CymaBay, added, "Our belief in the potential of seladelpar to transform patient treatment by improving measures of disease activity, reducing symptom related difficulties is bolstered by the RESPONSE results. We find these outcomes in line with our previous discoveries in what we are convinced has been an extraordinarily solid PBC development program. We have confidence in the uniquely beneficial role of the delpar mechanism for normalizing cholestasis indicators along with itch alleviation."
RESPONSE is an global, double-blind, placebo-controlled study for one year that randomly assigned 193 PBC patients to seladelpar 10 mg or a placebo in a 2:1 ratio, and administered once daily. Qualified participants demonstrated an inadequate reaction or inability to tolerate ursodeoxycholic acid, having serum alkaline phosphatase levels 1.67x above the upper normal limit despite a minimum treatment period of 12 months.
The primary evaluation criterion is the respondent ratio determined by a patient who has achieved an ALP level below the 1.67x upper normal limit with a minimum 15% decline in ALP and a total bilirubin of 1.0x below the upper normal limit after 52 weeks.
Each patient's proportion with ALP level equal or less than 1.0x the upper normal limit at 12 months & the changes from the initial evaluation after 6 months in the level of pruritus reported by the patient, judged by the NRS in those patients with initial NRS ≥4 were the secondary evaluation standards. NRS is a 0 to 10 scale. At the beginning of the study, the average ALP level was 314.3 U/L and TB 0.76 mg/dL and the mean baseline NRS was 6.3 in those patients assessed for the formal pruritus endpoint. Baseline features were even among the two groups and typical of a PBC patient population with high risk and a high burden of symptoms.
Seladelpar is a prospective treatment for people with PBC. It's a novel oral, selective peroxisome proliferator-activated receptor delta agonist, or delpar, which has been shown to control major metabolic and liver disease pathways in areas lacking medical solutions. Preclinical and clinical studies back its ability to regulate genes related to bile acid synthesis, inflammation, fibrosis, and lipid metabolism, storage, and transport.
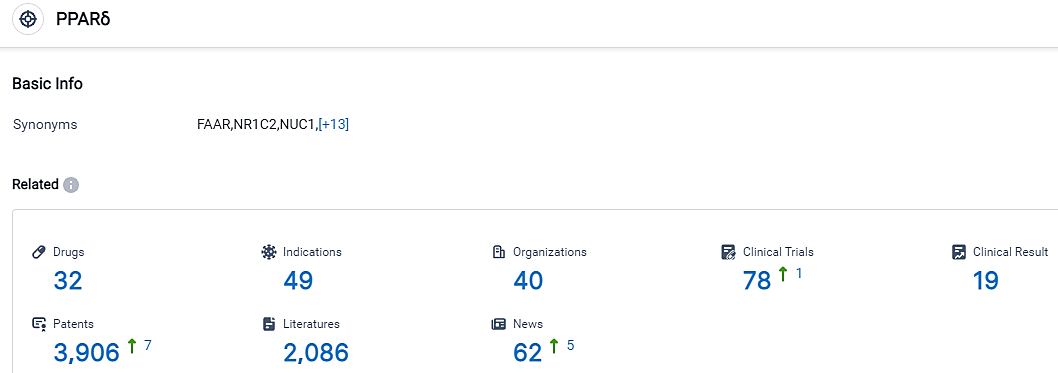
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 12, 2023, there are 32 investigational drugs for the PPARδ target, including 49 applicable indications, 40 R&D institutions involved, with related clinical trials reaching 78,and as many as 3906 patents.
Seladelpar, an investigational treatment for people with PBC, is a first-in-class oral, selective peroxisome proliferator-activated receptor delta agonist, or delpar. The drug has received regulatory designations such as PRIME, orphan drug, and breakthrough therapy, highlighting its potential therapeutic benefits and the recognition it has received from regulatory authorities.