1.0 mg Semaglutide Reduces Kidney Disease Events by 24% in Type 2 Diabetics with Chronic Conditions
Novo Nordisk recently disclosed the principal findings from their renal trial, referred to as FLOW. This revelation comes after an earlier announcement on October 10, 2023, when the decision was made to cease the trial prematurely because the desired results were effectively met. This decision was founded on counsel provided by a separate Independent Data Monitoring Committee.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
A clinical study utilizing a double-masked design assessed the effects of administered semaglutide at a dose of 1.0 mg alongside a placebo, augmenting the standard care regimen aimed at mitigating the advancement of renal dysfunction and lessening the occurrence of renal and cardiac-related fatalities in subjects with type 2 diabetes and chronic kidney disease (CKD). This research included a total of 3,533 individuals diagnosed with type 2 diabetes and CKD.
The study successfully met its key objective, showcasing a significant and more pronounced decrease of 24% in the escalation of renal disease and associated mortality due to cardiac and renal causes among participants receiving the semaglutide 1.0 mg treatment, in comparison to those given the placebo.
The integrated primary outcome was composed of five individual measures that gauged the advancement of CKD and the associated mortality risks of both renal and cardiac origin. The reduction in risk was attributed to the improvement seen in both the CKD and cardiac aspects defined within the primary outcome. Additionally, the dominance of semaglutide 1 mg over the placebo received confirmation through the secondary endpoints that were pre-specified for validation.
Throughout the study, semaglutide at the dosage of 1.0 mg maintained a safety and tolerability profile consistent with outcomes observed in preceding trials of the same dosage.
Martin Holst Lange, the Executive Vice President for Development at Novo Nordisk, expressed significant optimism about the findings from the FLOW study declaring, “Semaglutide at 1.0 mg presents us with a substantial stride forward in diminishing the jeopardy of renal diseases' progression,” and he noted the relevance of these findings for approximately 40% of individuals with type 2 diabetes who also suffer from CKD, signaling the glaring potential for semaglutide to emerge as a pioneering GLP-1 therapeutic alternative for this demographic.
Looking ahead, Novo Nordisk anticipates pursuing applications for extended indications of Ozempic in both the United States and European markets come 2024. The exhaustive results stemming from the FLOW trial are slated for disclosure at a medicinal conference planned for 2024.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
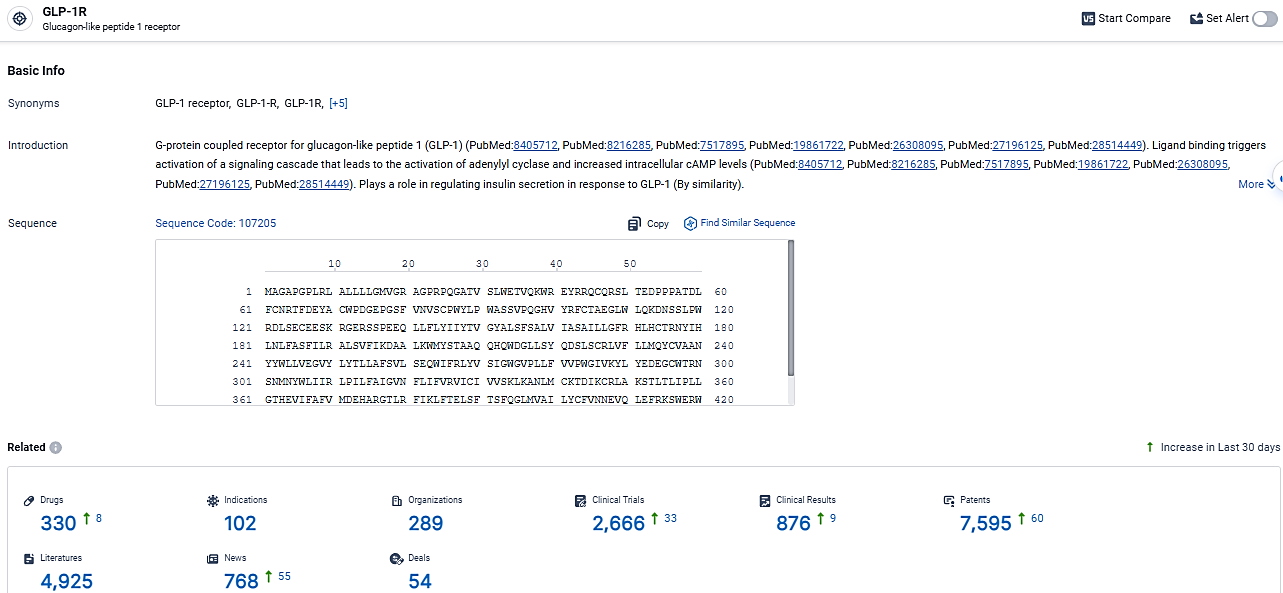
According to the data provided by the Synapse Database, As of March 7, 2024, there are 330 investigational drugs for the GLP-1R target, including 102 indications, 289 R&D institutions involved, with related clinical trials reaching 2666, and as many as 7595 patents.
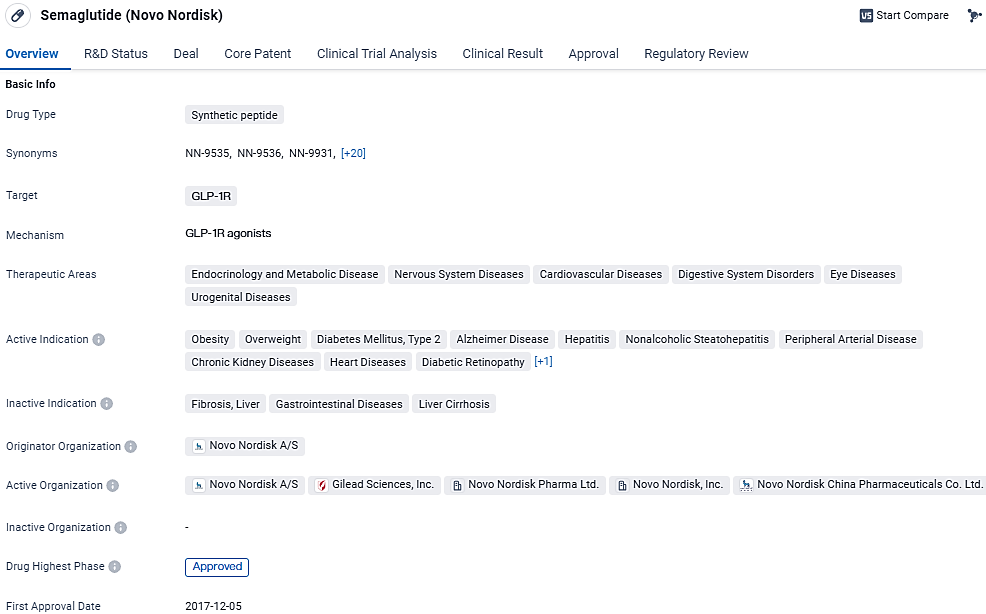
semaglutide targets the GLP-1R receptor and has been approved for various therapeutic areas, including endocrinology and metabolic disease, nervous system diseases, cardiovascular diseases, digestive system disorders, eye diseases, and urogenital diseases. Semaglutide received its first approval in the United States in December 2017 and has also been approved in China. Its breakthrough therapy designation highlights its potential to address unmet medical needs.