Weekly Islatravir-Lenacapavir Combo Maintains Viral Control in Phase 2 Trial
Gilead Sciences, Inc. and Merck, known as MSD outside of the United States and Canada have publicly shared the outcomes of a Phase 2 clinical trial. This study focused on the assessment of a novel treatment regimen which includes islatravir, a nucleoside reverse transcriptase translocation inhibitor currently under investigation, in combination with lenacapavir, an innovative agent classified as a capsid inhibitor.
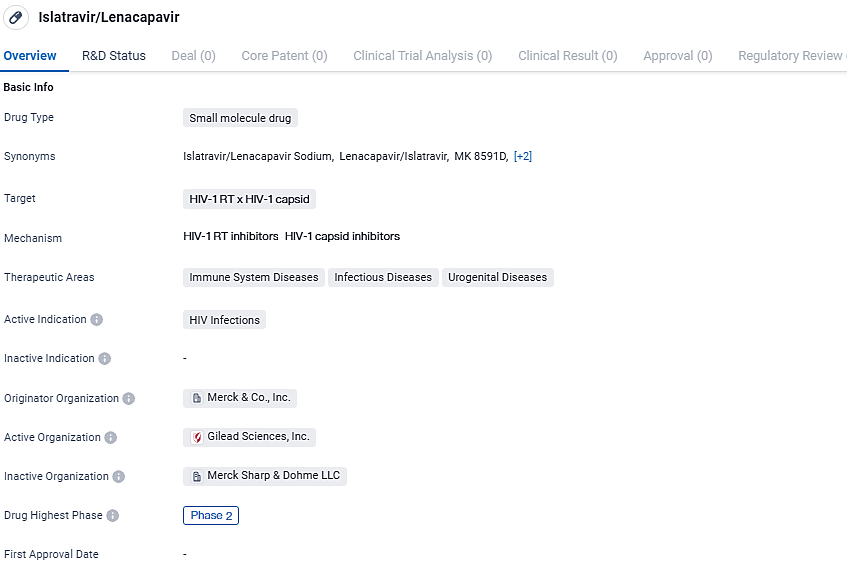
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
These freshly revealed findings were disclosed in an oral segment at the 31st Conference on Retroviruses and Opportunistic Infections. Before this engaging oral exposition, a press briefing by CROI highlighted the crucial outcomes.
After a 24-week period, the test combination under investigation successfully sustained elevated levels of reduced viral activity, which constitutes an ancillary goal of this research. Evaluation of the main objective revealed that, at week 24, a subject receiving islatravir alongside lenacapavir presented with a viral count exceeding 50 copies/mL; however, by week 30, the virus was undetectable in the individual while continuing the same treatment regimen.
The robust antiretroviral efficacy, fused with the pharmacokinetic characteristics of islatravir and lenacapavir, advocate for the advancement of this innovative once-a-week oral regimen for further investigations. Daily oral treatments administered via a single pill have significantly advanced HIV treatment, yet therapies that require less frequent administration could contribute to surmounting the hurdles of adherence and discrimination, among other issues, for certain individuals on daily antiretroviral therapies.
"Tailoring HIV treatments to individual needs is essential - the exploration of weekly treatment alternatives could address personal requirements, aiming for the enhancement of durable outcomes for those living with HIV," remarked Jared Baeten, who is the Vice President of HIV Clinical Development at Gilead Sciences, with both an MD and a PhD. "The encouraging data shared at CROI takes us a step nearer to achieving an array of choices that might revolutionize the field of HIV therapy."
Islatravir, as a solo agent or when paired with lenacapavir, remains in the experimental stage and lacks authorization across the globe. The efficacy and security of the islatravir and lenacapavir duo are yet to be certified.
Lenacapavir, sold under the brand name Sunlenca, has received authorization in several countries including Australia, Canada, the European Union, Israel, Japan, Switzerland, United Arab Emirates, United Kingdom, and the United States, specifically for treating individuals with HIV that shows resistance to multiple drugs, as part of a combined antiretroviral therapy.
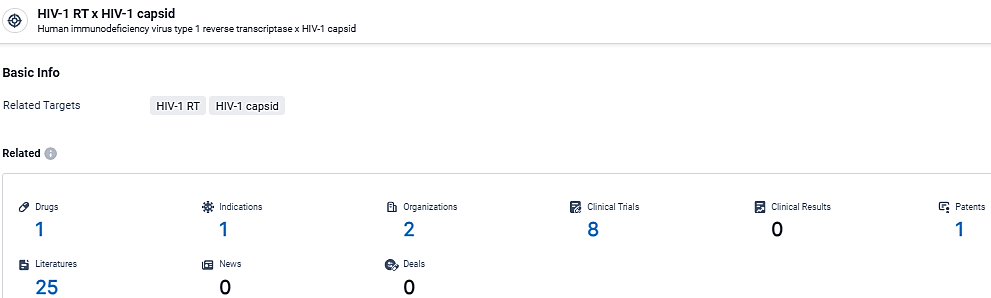
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 7, 2024, there are 1 investigational drugs for the HIV-1 RT and capsid target, including 1 indications,2 R&D institutions involved, with related clinical trials reaching 8, and as many as 1 patents.
Islatravir/Lenacapavir is a small molecule drug that shows promise in targeting HIV-1 RT and capsid. With its active indication being HIV infections and its current status in Phase 2 of development, this drug has the potential to address a significant unmet medical need in the field of biomedicine. Further clinical trials and research will be necessary to determine its safety and efficacy, but the initial information suggests that Islatravir/Lenacapavir could be a valuable addition to the treatment options available for HIV infections.