Senti Bio Initiates Phase 1 Trial of SENTI-202 for Recurrent or Treatment-Resistant Blood Cancers
Senti Biosciences, Inc., which specializes in advancing novel cell and gene therapies through its unique Gene Circuit technology, has initiated the first patient dosing in its Phase 1 clinical study for SENTI-202. This trial targets the treatment of relapsed or refractory hematologic malignancies, such as acute myeloid leukemia.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
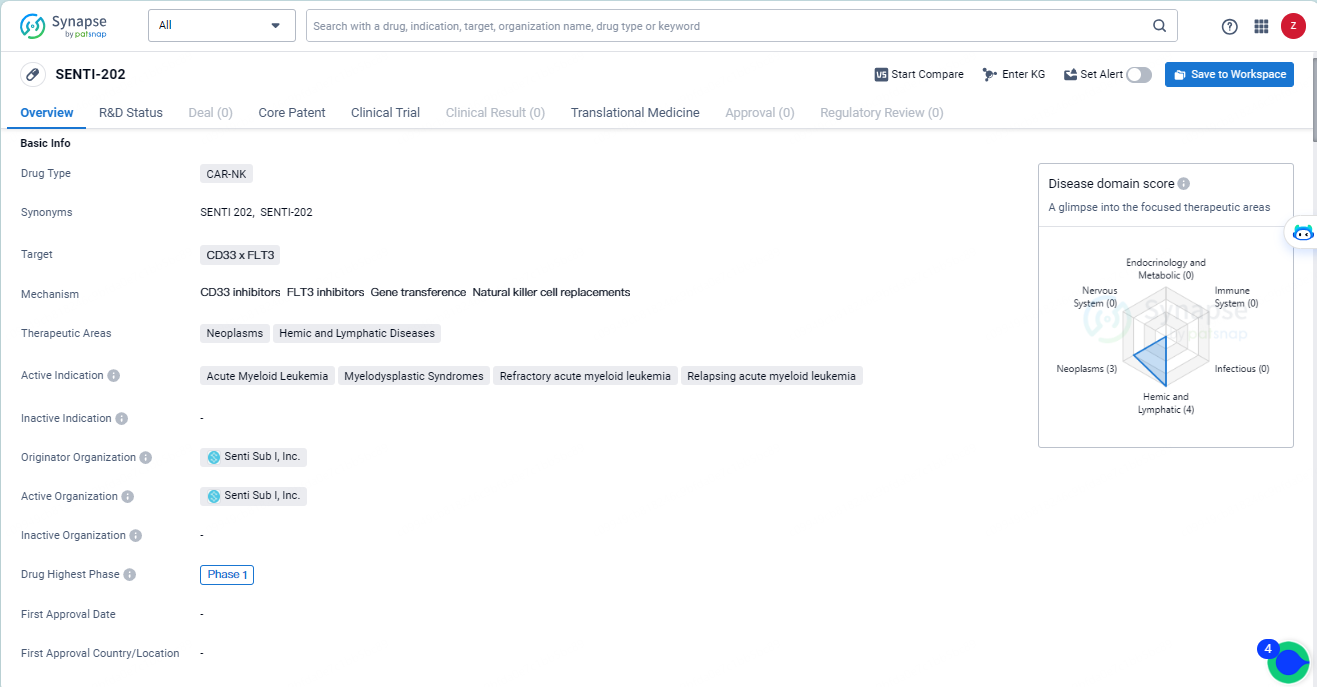
AML, a predominant type of acute leukemia affecting adults, emerges from the blood and bone marrow. SENTI-202, heralded as a revolutionary Logic Gated off-the-shelf chimeric antigen receptor natural killer investigational cell therapy, aims to precisely eliminate cells expressing CD33 and/or FLT3 involved in hematologic malignancies like AML, while preserving the cells of healthy bone marrow. The project expects to release initial efficacy results by the close of 2024, with durability findings to follow in 2025.
"The initiation of the Phase 1 clinical trial for SENTI-202 is a pivotal advancement in our commitment to reshape treatment paradigms for AML patients who face limited options and poor prognoses. Our meticulous approach in developing SENTI-202 to navigate the complex variance in AML and to protect viable marrow cells is poised to overcome the shortcomings seen in existing treatments," expressed Kanya Rajangam, MD, PhD, who serves as the Head of Research & Development and Chief Medical Officer at Senti Bio.
The ongoing Phase 1 trial of SENTI-202 is currently admitting adult participants with recurrent or resistant CD33 and/or FLT3 expressing hematologic malignancies, including AML, across various locations in the United States and Australia. This dose-escalation study is assessing two quantities, either 1 or 1.5 billion SENTI-202 cells, with administration occurring in cycles of three weekly doses following a disease-specific lymphodepleting preparatory regimen. Based on determinations of safety and efficacy, patients have the option to undergo subsequent cycles.
SENTI-202, a novel Logic Gated off-the-shelf CAR-NK cell therapy candidate, focuses on the selective eradication of CD33 and/or FLT3 expressing hematologic malignancies, such as AML and myelodysplastic syndrome, whilst sparing healthy bone marrow cells. SENTI-202 comprises three principal elements:
Initially, the OR GATE, an active CAR that targets CD33 and FLT3. By engaging with either or both antigens, SENTI-202 has the potential to destroy both leukemic blasts and the critical leukemic stem cells that are central to the progression of AML. Additionally, the NOT GATE is developed to distinguish and shield healthy cells from destruction. Lastly, the innovative calibrated-release IL-15 technology is incorporated to markedly enhance the persistence, proliferation, and effectiveness of both the CAR-NK cells and the native immune cells.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
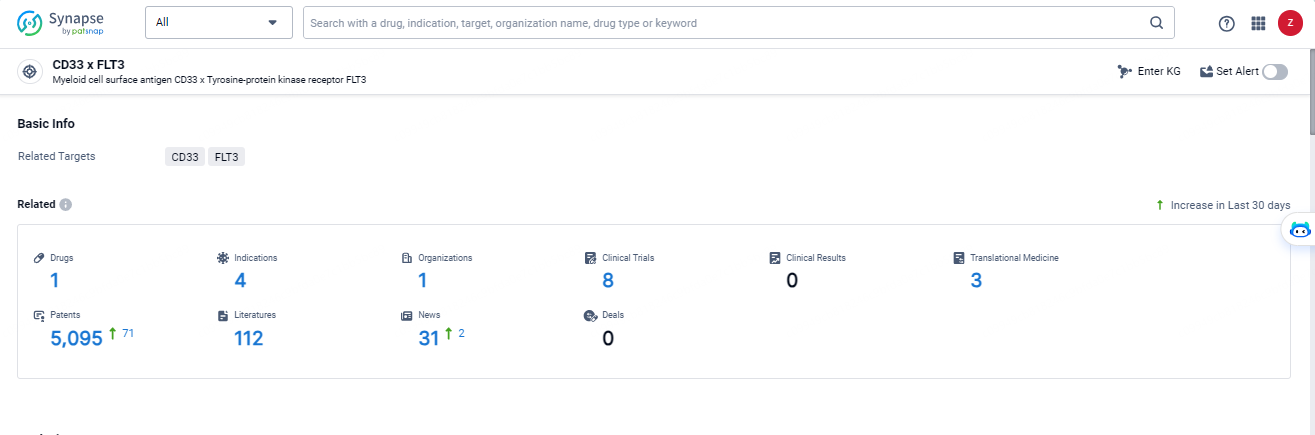
According to the data provided by the Synapse Database, As of May 14, 2024, there are 1 investigational drugs for the CD33 and FLT3 target, including 4 indications, 1 R&D institutions involved, with related clinical trials reaching 8, and as many as 5095 patents.
SENTI-202 targets CD33 x FLT3 and is indicated for the treatment of AML, MDS, refractory AML, and relapsing AML. Currently in Phase 1, the drug holds promise as a potential therapeutic option for patients with these conditions, but further research and clinical trials are needed to establish its safety and efficacy.