Silence Therapeutics Reports Positive 48-Week Phase 2 Results of Zerlasiran in Elevated Lipoprotein(a) Patients
Silence Therapeutics plc, Nasdaq: SLN, an experienced and innovative biotechnology company committed to transforming people’s lives by silencing diseases through precision engineered medicines, announced positive topline 48-week data from the ALPACAR-360 phase 2 study of zerlasiran (SLN360) in 178 subjects with baseline lipoprotein, or Lp, levels at or over 125 nmol/L at high risk of atherosclerotic cardiovascular disease events.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
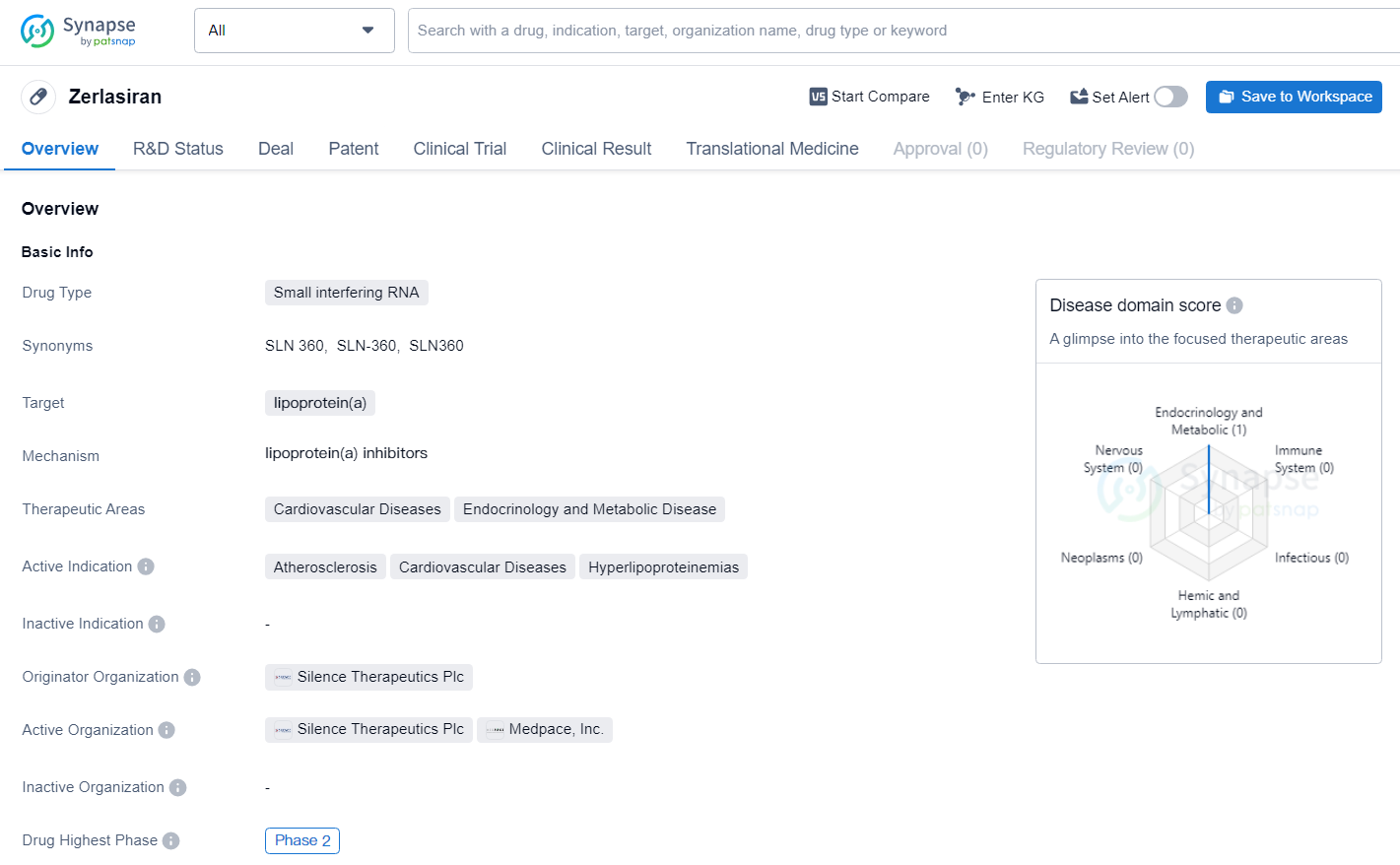 Zerlasiran is a siRNA designed to lower the body’s production of Lp, a key genetic risk factor for cardiovascular disease affecting up to 20% of the world’s population.
Zerlasiran is a siRNA designed to lower the body’s production of Lp, a key genetic risk factor for cardiovascular disease affecting up to 20% of the world’s population.
In the double-blind placebo-controlled treatment period, zerlasiran was administered at 300 mg subcutaneously every 16 or 24 weeks and 450 mg every 24 weeks to patients with a median baseline Lp of approximately 215 nmol/L. These data demonstrated a highly significant reduction from baseline in Lp compared to placebo to 48 weeks. Median maximum Lp reduction of approximately 90% or greater was observed for both doses during the treatment period.
Zerlasiran was well tolerated with no serious safety concerns. As previously announced, the study met its primary endpoint with a highly significant reduction from baseline in Lp compared to placebo to 36 weeks. The study is ongoing, and patients will be followed through to week 60.
“We are encouraged by the strength of the phase 2 data and emerging competitive profile of zerlasiran, which support an infrequent dosing regimen of at least quarterly with the 300 mg dose,” said Steven Romano, MD, Head of Research and Development at Silence. “We look forward to advancing zerlasiran to phase 3 as a potential treatment for this major unmet need in cardiovascular disease.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
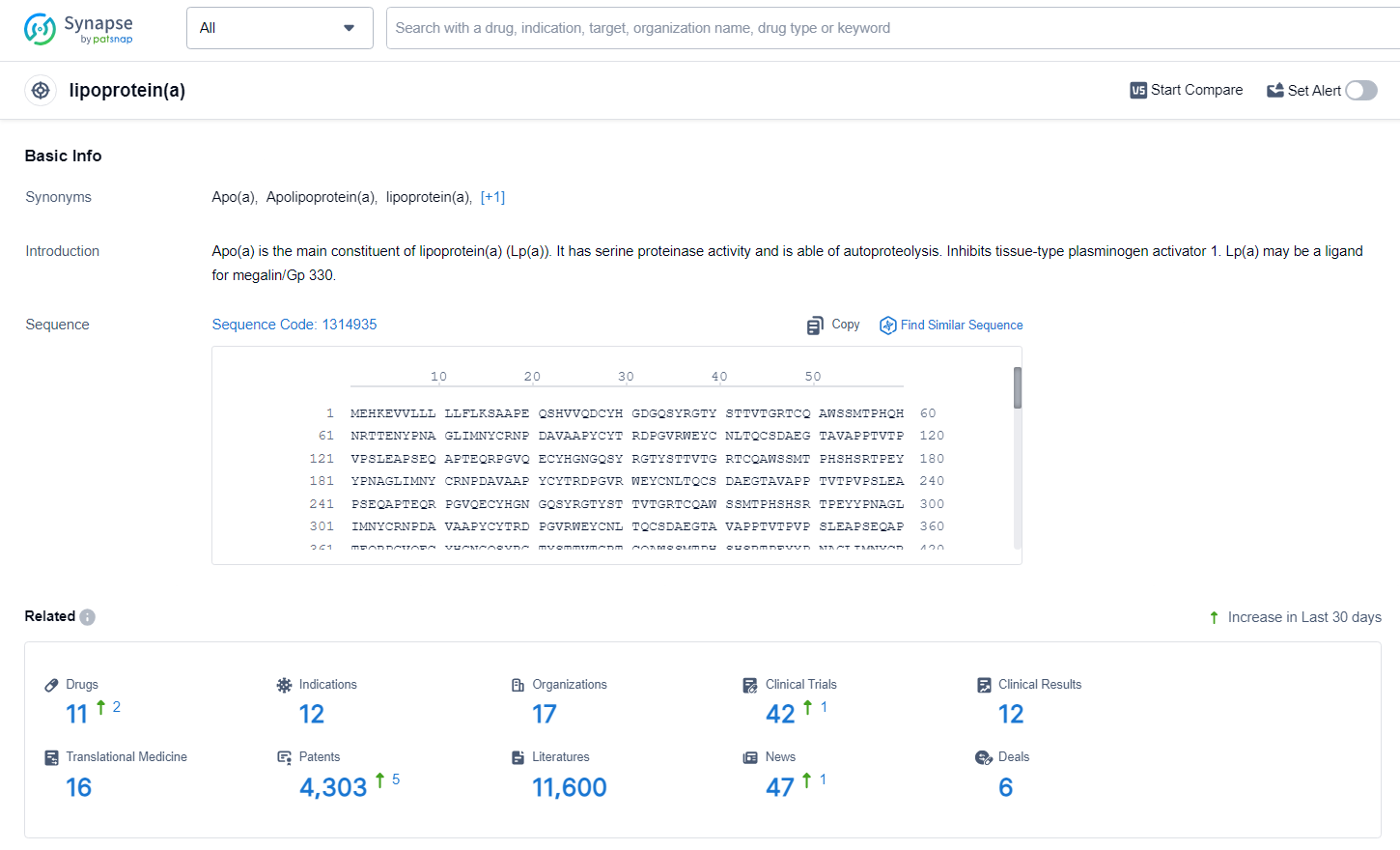
According to the data provided by the Synapse Database, As of June 24, 2024, there are 11 investigational drugs for the lipoprotein(a) target, including 12 indications, 17 R&D institutions involved, with related clinical trials reaching 42, and as many as 4303 patents.
Zerlasiran targets lipoprotein(a), a known risk factor for cardiovascular diseases. As with any drug in clinical development, further research and testing are necessary to fully evaluate the safety and efficacy of Zerlasiran. However, its progression to Phase 2 trials indicates that it has shown promise in early studies, and its development is worth monitoring for potential future advancements in the treatment of cardiovascular diseases and related conditions.