Is Zanubrutinib approved by the FDA?
Yes, Zanubrutinib, marketed under the brand name Brukinsa, is FDA approved. The U.S. Food and Drug Administration (FDA) approved Zanubrutinib on November 14, 2019. This approval was for the treatment of certain B-cell cancers, specifically mantle cell lymphoma (MCL) in patients who have received at least one prior treatment.
What is Zanubrutinib?
Zanubrutinib is a second-generation Bruton tyrosine kinase (BTK) inhibitor. It works by blocking the BTK protein, which plays a crucial role in the growth and multiplication of B-cells. By inhibiting this protein, Zanubrutinib helps to stop the proliferation of cancerous B-cells.
Indications and Usage
Zanubrutinib is prescribed for the treatment of adults with:
- Chronic Lymphocytic Leukemia or Small Lymphocytic Lymphoma (CLL/SLL)
- Waldenström's Macroglobulinemia (WM)
- Mantle Cell Lymphoma (MCL): For patients who have received at least one prior treatment.
- Marginal Zone Lymphoma (MZL): For patients whose cancer has returned or did not respond to treatment after at least one anti-CD20-based regimen.
- Relapsed or Refractory Follicular Lymphoma (FL): In combination with obinutuzumab, after two or more lines of systemic therapy.
Dosage and Administration
Zanubrutinib is available in oral capsule form, with the recommended dosage being 160 mg taken twice daily or 320 mg once daily. The capsules should be swallowed whole with water and can be taken with or without food. Patients with severe hepatic impairment may require a reduced dose.
Side Effects
Common Side Effects
- Decreased white blood cells
- Upper respiratory tract infection
- Decreased platelet count
- Bleeding
- Rash
- Muscle or joint pain
Serious Side Effects
- Bleeding problems (hemorrhage): Increased risk if taken with blood thinners.
- Infections: Can be serious and potentially fatal.
- Decreased blood cell counts: Including white blood cells, platelets, and red blood cells.
- Second primary cancers: Including skin cancers and other organ cancers.
- Heart rhythm problems: Such as atrial fibrillation and atrial flutter.
Warnings and Precautions
Patients should inform their healthcare provider about any medical conditions before starting Zanubrutinib, especially if they have:
- Bleeding problems
- Recent or planned surgeries
- Infections
- Heart rhythm problems
- High blood pressure
- Liver problems, including hepatitis B
Pregnancy and Breastfeeding
Zanubrutinib can harm an unborn baby. Women who can become pregnant should use effective birth control during treatment and for at least one week after the last dose. Men should also use effective contraception during treatment and for at least one week after the last dose. Breastfeeding is not recommended during treatment and for two weeks after the last dose.
Storage
Zanubrutinib capsules should be stored at room temperature, between 68°F to 77°F (20°C to 25°C), in a bottle with a child-resistant cap. Keep the medication out of reach of children.
Conclusion
Zanubrutinib (Brukinsa) is an FDA-approved medication used to treat various B-cell cancers. Approved in November 2019, it offers a targeted therapy option by inhibiting Bruton's tyrosine kinase, crucial for B-cell growth. Patients should follow their healthcare provider's instructions and be aware of potential side effects and necessary precautions.
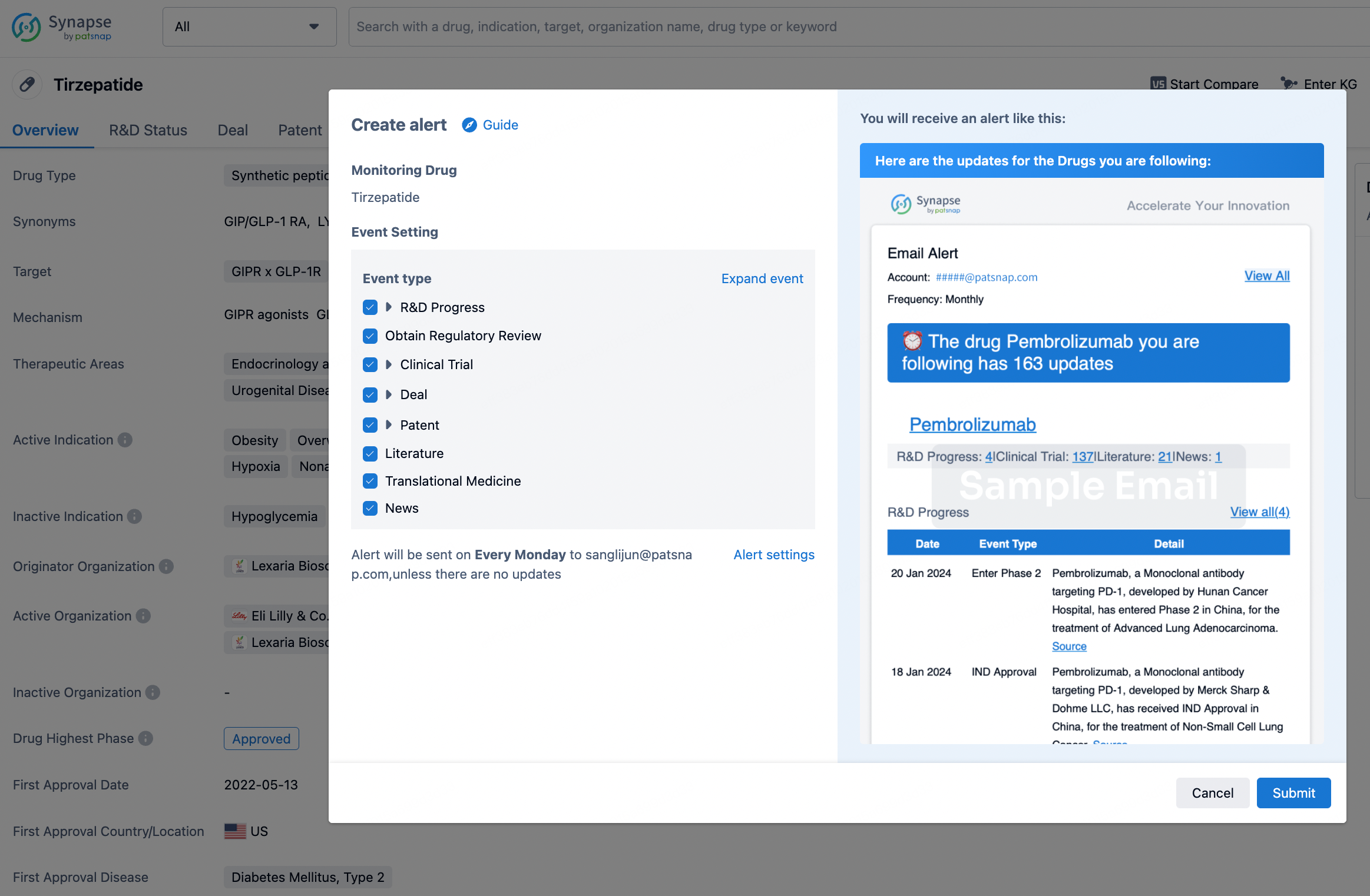
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!