Sionna Therapeutics Starts Two Phase 1 Clinical Trials with SION-719 and SION-451 for Cystic Fibrosis
Sionna Therapeutics, a life sciences company at the clinical stage focusing on creating advanced and unique treatments for cystic fibrosis, stated that the initial participants have received doses in two distinct Phase 1 clinical trials aimed at assessing the safety, tolerability, and pharmacokinetics of SION-719 and SION-451 in healthy individuals.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
SION-719 and SION-451 are investigational therapies from the company’s new generation of highly potent nucleotide-binding domain 1 (NBD1) stabilizers. These trials are taking place in Australia under the Clinical Trial Notification procedure.
“We are delighted with the Company’s robust performance and the progress these pioneering programs have achieved by entering clinical trials,” said Mike Cloonan, President and CEO of Sionna. “We possess a unique capability to target NBD1, which we believe could significantly enhance efficacy and provide better clinical benefits for CF patients. The data from these single and multiple ascending dose studies will guide our choice of a lead NBD1 stabilizer for the next phases of clinical development."
CF results from mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. This gene encodes for an epithelial ion channel crucial for producing healthy, free-flowing mucus in the airways, digestive tract, and other organs. The most prevalent mutation in CFTR, F508del, leads to the unfolding of NBD1 at body temperature, substantially reducing CFTR function.
Sionna has shared preclinical data, including findings from the clinically predictive human bronchial epithelial cell model, indicating that its NBD1 stabilizers, SION-719 and SION-451, significantly improve CFTR protein activity in vitro when paired with complementary modulators, showing potential for superior clinical outcomes. Sionna is pioneering the development of the first clinical-stage NBD1 stabilizers and is also working on complementary modulators to advance distinctive combination therapies for CF.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
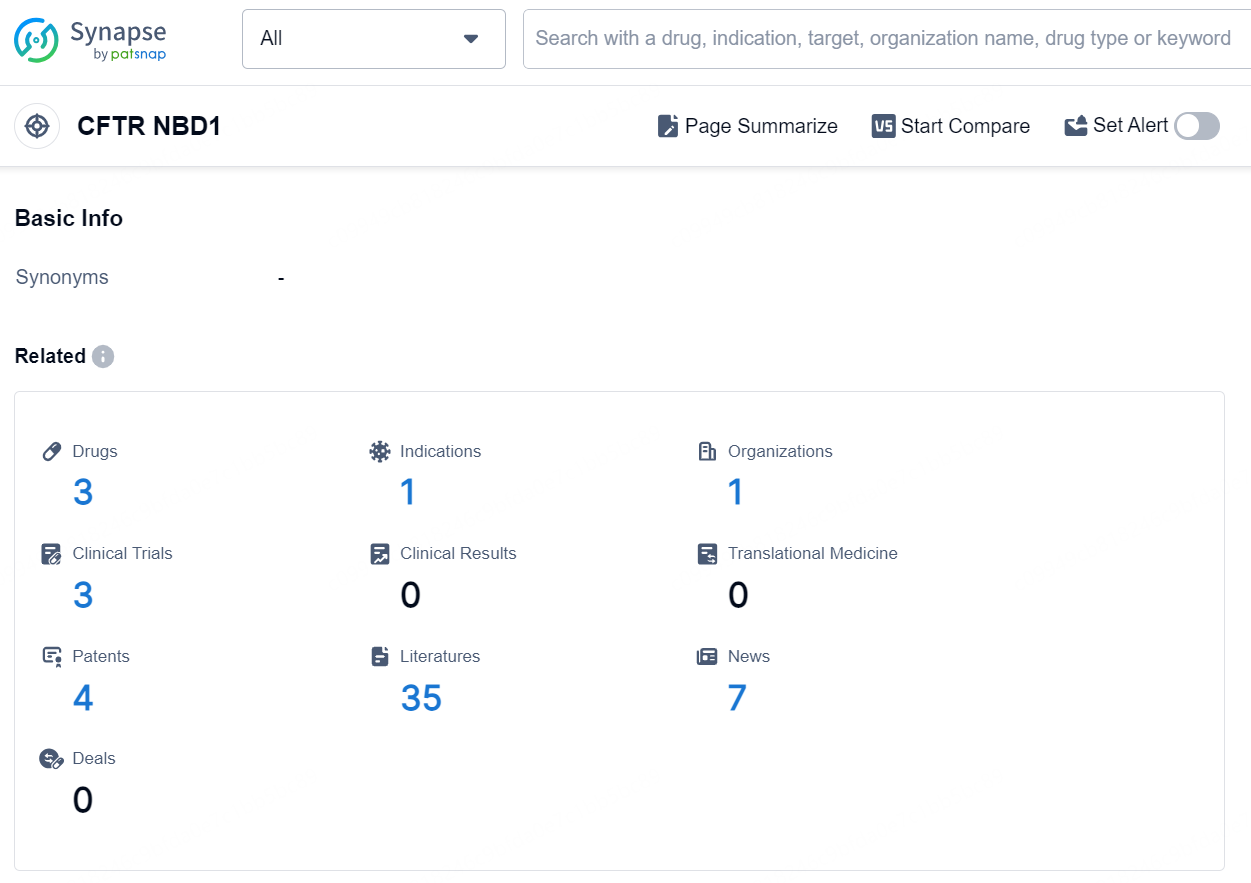
According to the data provided by the Synapse Database, As of August 20, 2024, there are 3 investigational drugs for the CFTR NBD1 target, including 1 indication, 1 R&D institution involved, with related clinical trials reaching 3, and as many as 4 patents.
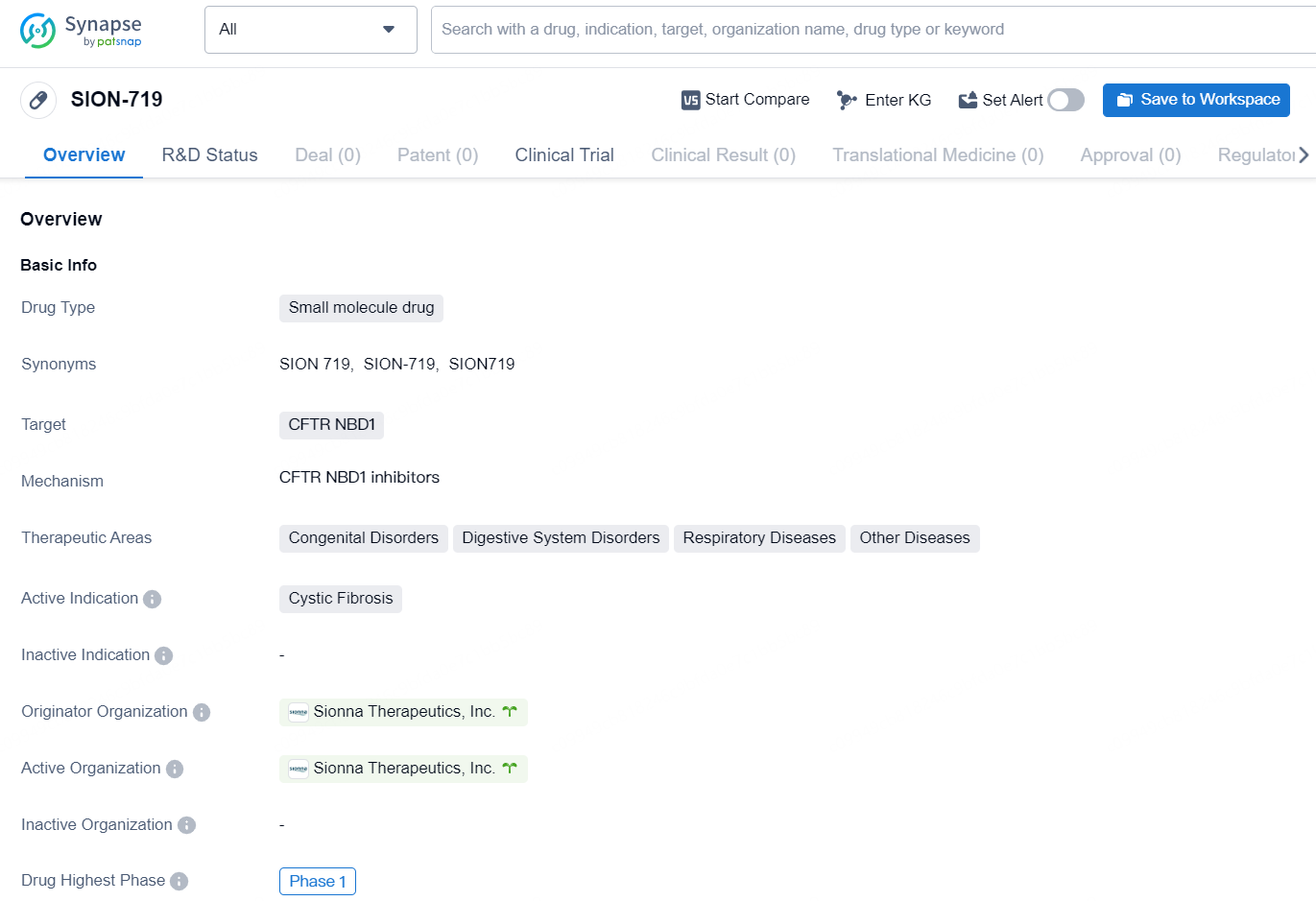
SION-719 is a small molecule drug developed by Sionna Therapeutics, Inc. It targets the CFTR NBD1 and is primarily intended for the treatment of Cystic Fibrosis. The drug falls within the therapeutic areas of Congenital Disorders, Digestive System Disorders, Respiratory Diseases, and Other Diseases. SION-719 has reached the highest global phase of Phase 1 in its development. This indicates that the drug has undergone initial testing for safety, dosage, and potential side effects in a small group of people to evaluate its efficacy and to determine its optimal usage.