siRNA Drug Amvuttra Patent Research and Practical Operation Guide(1)
Small interfering RNA (siRNA), also known as silencing RNA, short interfering RNA, or non-coding RNA, is a class of short double-stranded RNA molecules of 21 to 25 nucleotides in length that has proven to be a promising therapeutic modality. siRNA is expected to become a basic method for the treatment of various infectious diseases, neoplastic hematologic disorders, cardiovascular diseases, and neurodegenerative diseases due to its specificity and gene silencing function. Alnylam announced on June 14, 2022, that its siRNA drug Amvuttra® (vutrisiran) was approved by the FDA for the treatment of polyneuropathy with hereditary transthyretin-mediated amyloidosis (hATTR) in adults. Currently, more than 20 companies worldwide have entered and focused on the research and development of siRNA drugs, while Alnylam has a near monopoly on the siRNA market. High-quality drug patent research is particularly important for providing technical intelligence for enterprises' infringement analysis, biosimilar drug development, investment and financing, and drug R&D, while also helping to improve the core competitiveness of enterprises. The following guide will detail how to conduct prior art search for siRNA drugs through comprehensive and accurate patent retrieval and analysis.
Disclaimer:
The data presented in this report, primarily sourced from Patsnap Synapse, Bio, Chemical and Analytics databases, may exhibit discrepancies due to data source leakage, variations in statistical periods, and divergent search methodologies. Therefore, it should be regarded as a reference only. This report is not responsible for any business losses resulting from the usage of this information.
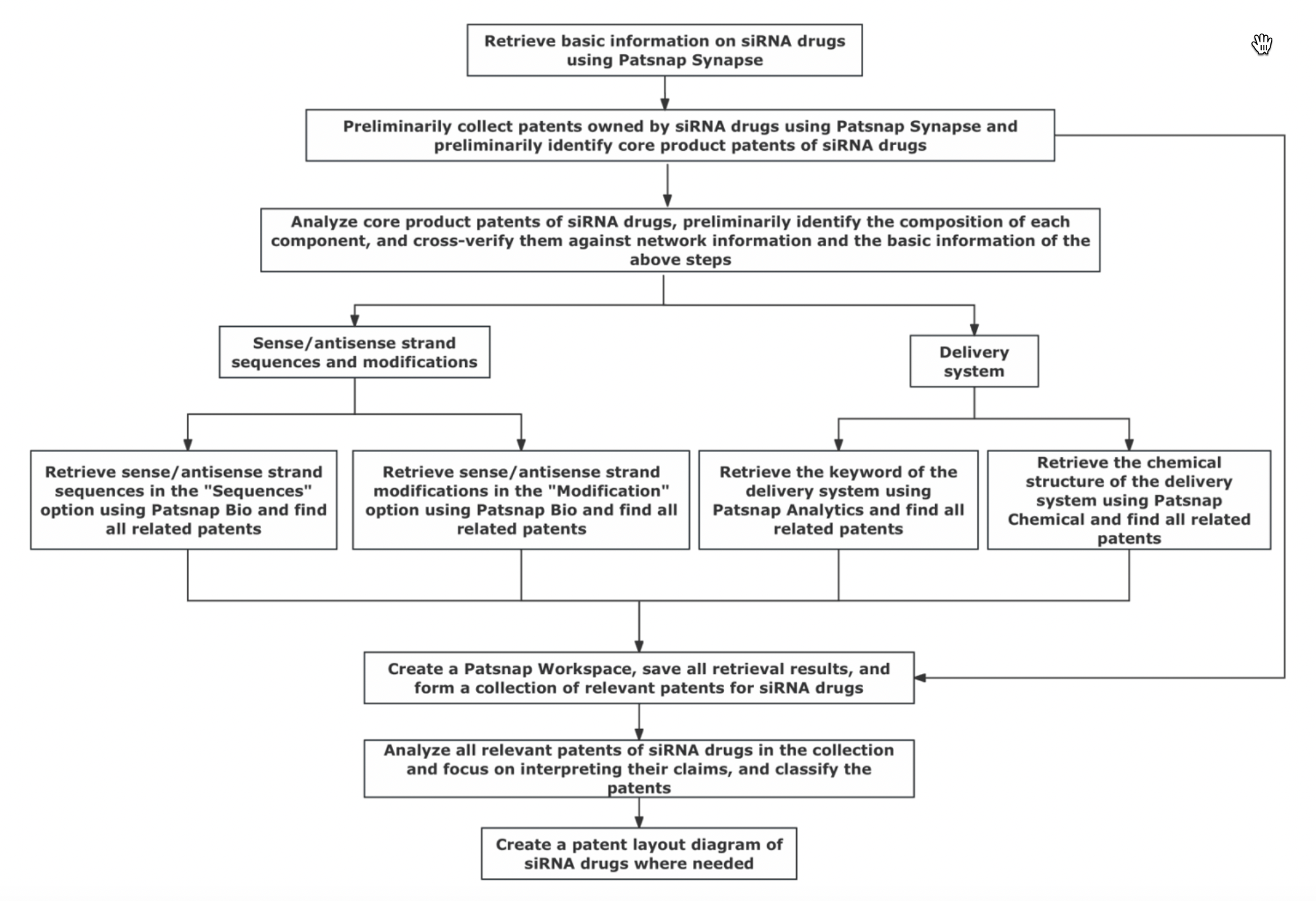
Based on the characteristics of siRNA drugs, we have summarized the process for conducting patent research on siRNA drugs. You can follow the process described below to carry out patent research on siRNA drugs.
For more information, please click the image link below to access the full report.




