D&D Pharmatech Initiates Phase 2 Trial of DD01 for Obesity-Related MASLD/MASH Treatment
D&D Pharmatech, Inc. (D&D) (KOSDAQ: 347850), a biotechnology company in the clinical-stage dedicated to developing disease-modifying drugs, announced that dosing has begun in a Phase 2 trial aimed at assessing the efficacy and safety of DD01 in overweight or obese individuals with metabolic dysfunction-associated steatotic liver disease (MASLD) or metabolic dysfunction-associated steatohepatitis (MASH). DD01 is a long-acting dual GLP-1/glucagon receptor agonist that has previously demonstrated rapid improvement in hepatic steatosis, glycemic control, and body weight reduction in subjects with fatty liver disease.
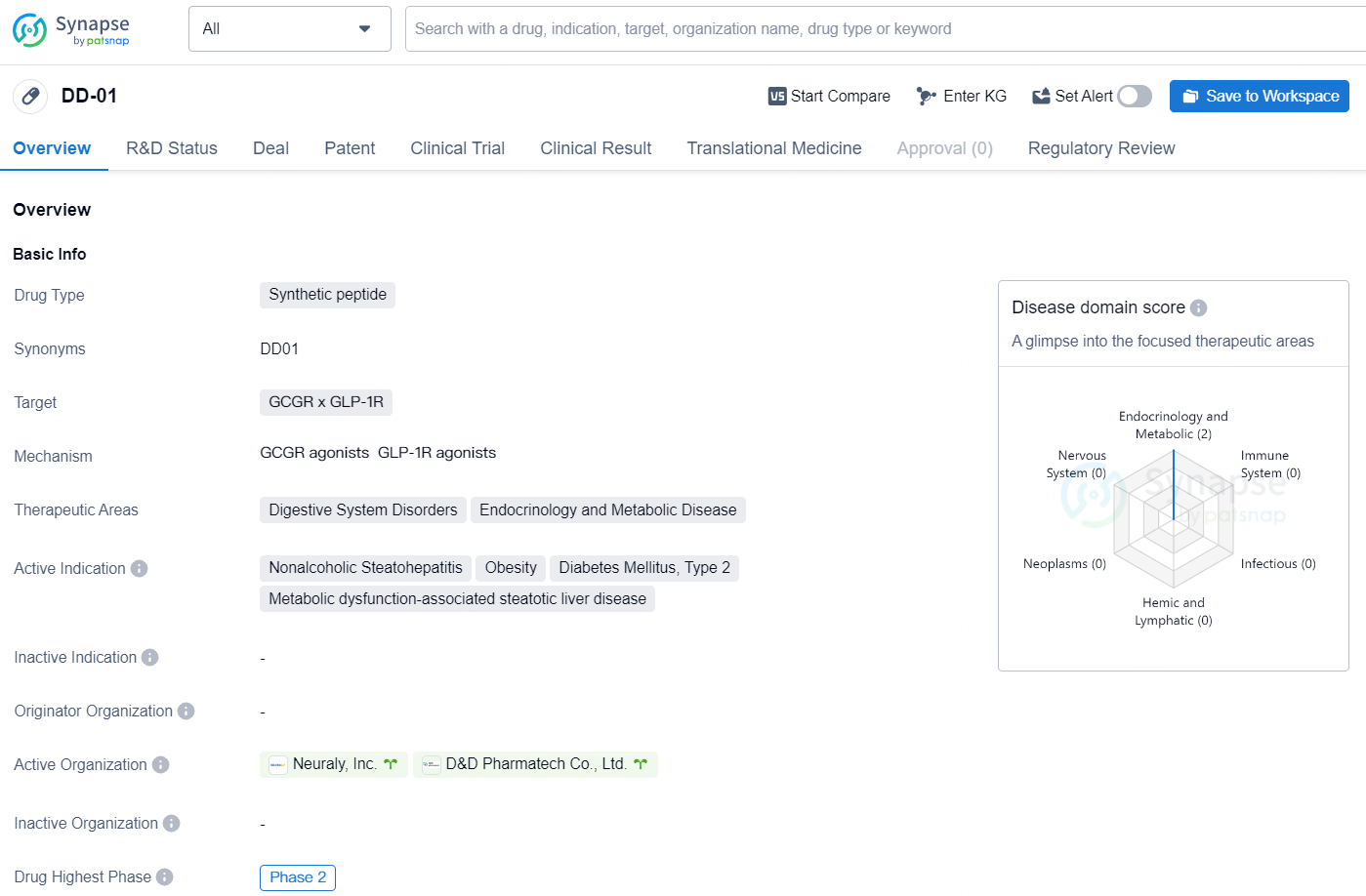
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
This Phase 2 study is a randomized, double-blind, placebo-controlled, biopsy-guided trial being executed at about 12 locations across the United States. Approximately 68 obese or overweight participants (BMI ≥25kg/m2) with biopsy-verified MASH or MASLD will be enrolled. Participants will be assigned in a 1:1 ratio to receive either 40 mg of DD01 or a placebo once weekly for 48 weeks. The main objective of the study is to determine the proportion of participants who achieve at least a 30% reduction in liver fat measured by MRI-PDFF from baseline to Week 12, with secondary and exploratory objectives assessing various additional safety and efficacy parameters, including MASH resolution, fibrosis improvement, HbA1c levels, and body weight, with dosing continuing throughout the 48-week period.
Earlier, D&D reported favorable safety and efficacy outcomes for DD01 in a Phase 1 multiple ascending dose (MAD) study in overweight or obese patients with type 2 diabetes (T2D) and MASLD. DD01 was generally found to be safe and well-tolerated at doses up to 80 mg weekly, and within just 4 weeks of treatment, up to 100% of patients experienced over 30% liver fat reduction as measured by MRI-PDFF, with a mean relative reduction exceeding 50% in liver fat content in a pooled analysis of the 40 mg and 80 mg doses. These swift improvements in steatosis were matched by reduced HbA1c in diabetic participants, lower levels of liver AST/ALT, and serum lipids. Throughout the 4-week treatment duration, subjects administered 40 and 80 mg of DD01 exhibited modest weight loss, which was not observed in placebo-treated individuals.
These trial outcomes are supported by preclinical studies in obese mice and primates demonstrating that DD01 treatment is more effective than either dietary changes or GLP-1 treatment alone in promoting liver fat reduction and weight loss. The lipolytic actions of glucagon are maintained and balanced with the anorectic and anti-diabetic properties of GLP-1, resulting in a true dual-pathway therapy. Resolution of MASH was observed in preclinical models, including enhancements in liver steatosis, lobular inflammation, hepatocyte ballooning, and fibrosis, which were accompanied by reductions. The US FDA has granted Fast Track designation to DD01 for the treatment of adults with MASLD/MASH.
“We are thrilled to announce the commencement of the DD01 Phase 2 study, marking a significant milestone in the development of DD01 for MASH treatment,” stated Seulki Lee, Ph.D., President and CEO of D&D Pharmatech. “Recent clinical investigations imply that dual activation of GLP-1 and liver-directed glucagon receptors would be more potent in treating MASH compared to GLP-1 analogs without glucagon agonism. Given that DD01 has already shown rapid and significant reductions in hepatic steatosis along with beneficial effects on glucose regulation within just 4 weeks of treatment, we anticipate achieving clinically meaningful rates of MASH resolution and fibrosis improvement in our Phase 2 trial.”
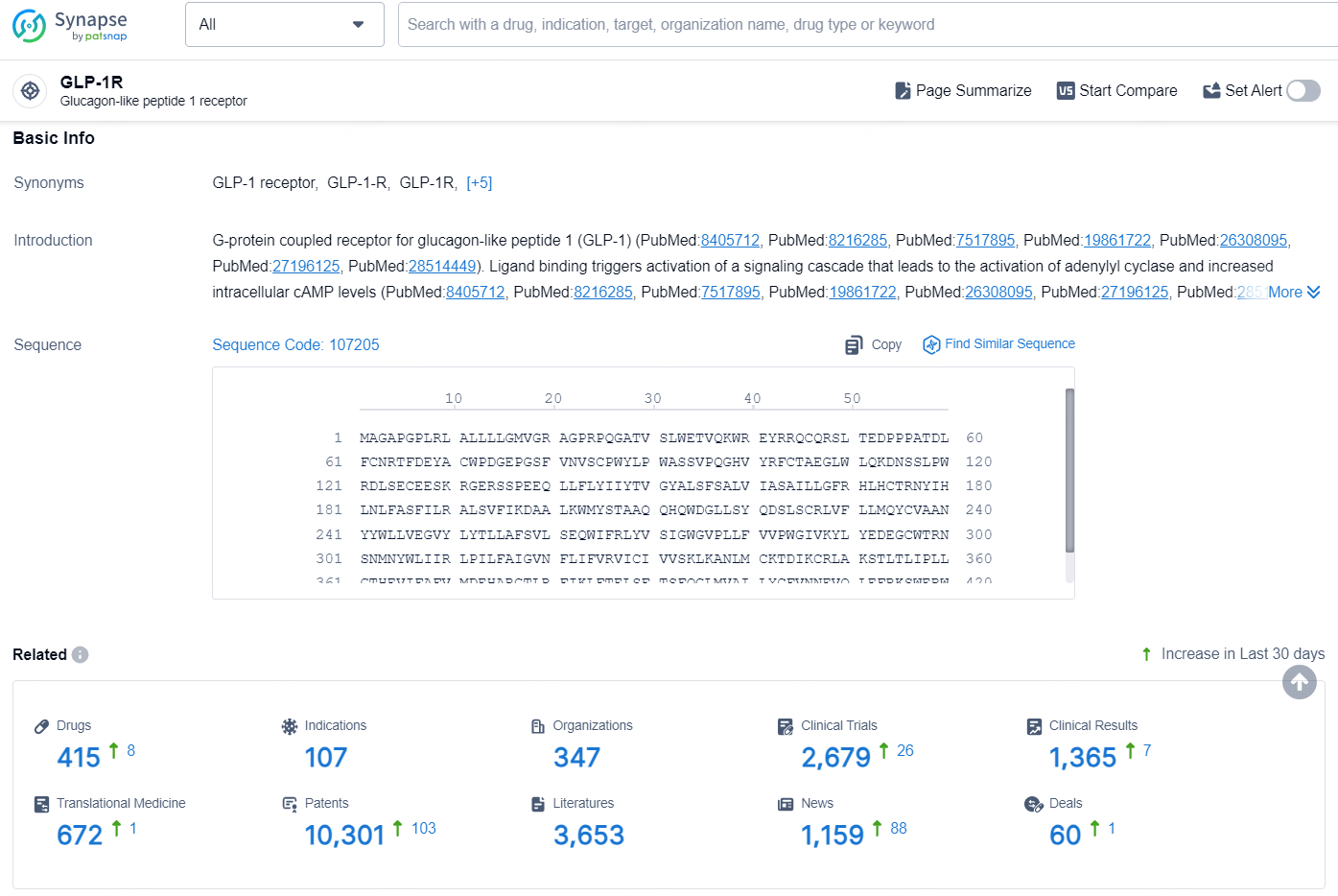
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 23, 2024, there are 415 investigational drugs for the GLP-1 targets, including 107 indications, 347 R&D institutions involved, with related clinical trials reaching 2679, and as many as 10301 patents.
DD01 is an exclusive, once-weekly dual agonist targeting GLP-1 (glucagon-like peptide-1) and glucagon receptors, boasting a half-life of 7-8 days in patients who are obese or overweight and suffering from T2D and MASLD. What sets DD01 apart is its dual action mechanism. Unlike other agonists, which typically act solely through the incretin pathway, DD01 enhances incretin therapy benefits through the liver-directed glucagon receptor, promoting liver lipolysis. This leads to swift effectiveness and the potential for liver-first treatment. Clinical studies show that DD01 significantly reduces liver fat in just 4 weeks in MAFLD patients. In preclinical trials, DD01 induced notable weight loss, decreased liver fat and fibrosis, and improved glucose tolerance across various models of obesity, diabetes, and fatty liver, including studies on non-human primates.