JAMP Pharma and Alvotech have been approved to sell Jamteki (AVT04), the first biosimilar of Stelara® (ustekinumab)
Alvotech, an international biotech corporation focusing on the production and progression of biosimilar drugs for global patients, and JAMP Pharma Group, a Canadian pharmaceutical company operating out of the Greater Montreal area, have declared that JAMP Pharma has received marketing approval from Health Canada for AVT04, a biosimilar to Stelara® (ustekinumab) produced by Alvotech. The marketing for AVT04 will be under the brand name Jamteki™.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
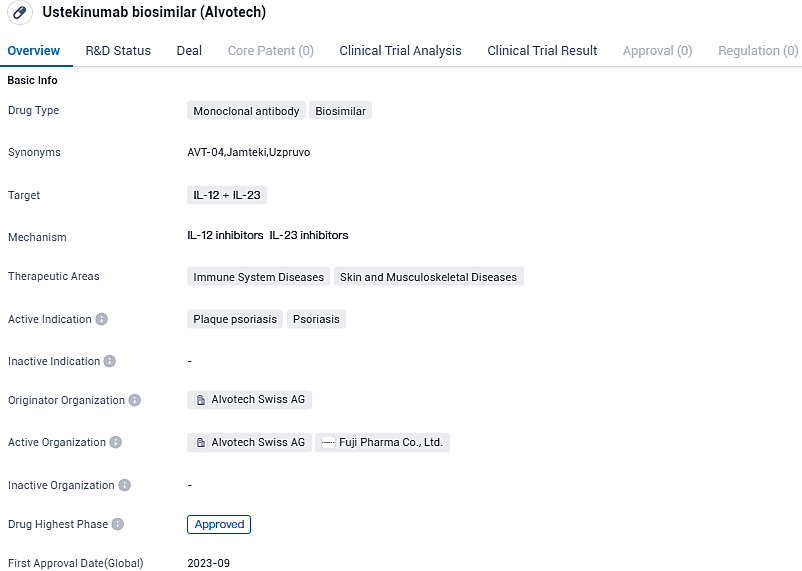
The existing approved versions of JamtekiTM include a 45mg/0.5mL syringe prefilled with a passive safety mechanism for injecting subcutaneously, and a 90mg/mL PFS-SD. This is the second biosimilar to be given marketing approval and was created through a unique commercialization partnership between Alvotech and JAMP Pharma. Previously, the collaborators introduced Simlandi®, a biosimilar for Humira® (adalimumab).
"Our launch of JamtekiTM aligns perfectly with our existing biosimilar products and highlights JAMP Pharma's commitment to advancing in this market sector," stated JAMP Pharma's CEO and President, Louis Pilon. "Alvotech, with its innovative and strategic methodologies, allows Canadian patients to access ustekinumab at a reduced price. Moreover, our Patient Support Program – JAMP CareTM will provide patients with expert guidance for a smooth transition into using the biosimilar."
"We're delighted about gaining marketing approval for our second biosimilar in Canada through our collaboration with JAMP Pharma," commented Robert Wessman, CEO and Chairman of Alvotech. "A high demand for biosimilars in Canada suggests the need for affordable, yet high-quality biologics within the Canadian market. This represents a significant milestone for us and aligns with our shared goal of offering wider access to cost-effective healthcare solutions."
In January 2022, JAMP Pharma and Alvotech established their special partnership for biosimilar commercialization in Canada, broadening the agreement in October 2022. Simlandi® (adalimumab), which was introduced in Canada in April 2022, as well as JamtekiTM (ustekinumab), are part of this collaboration. Additionally, four biosimilar products that are presently undergoing clinical trials and two that are in the pre-clinical trial phase are also included in the partnership.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
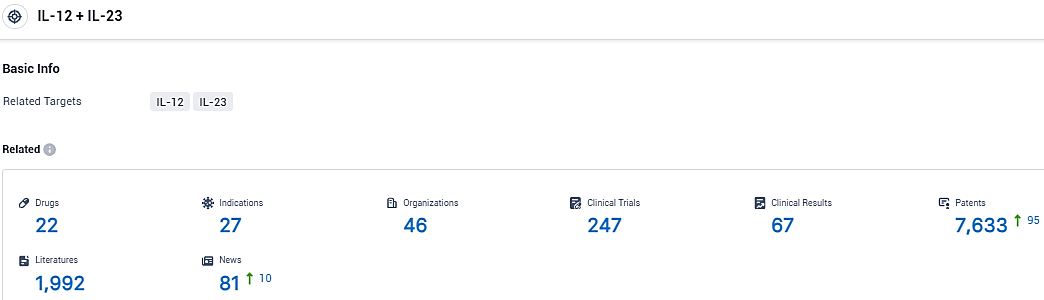
According to the data provided by the Synapse Database, As of November 22, 2023, there are 22 investigational drugs for the IL-12 and IL-23 target, including 27 indications, 46 R&D institutions involved, with related clinical trials reaching 247 and as many as 7633 patents.
AVT04 is a monoclonal antibody and a biosimilar of Stelara® (ustekinumab). Ustekinumab binds to two cytokines, IL-12 and IL-23, that are involved in inflammatory and immune responses. AVT04 has been approved in Japan, Canada and EMA has recommended market authorization in the 27 member states of the European Union, as well as Norway, Iceland and Liechtenstein, pending a final decision by the European Commission.