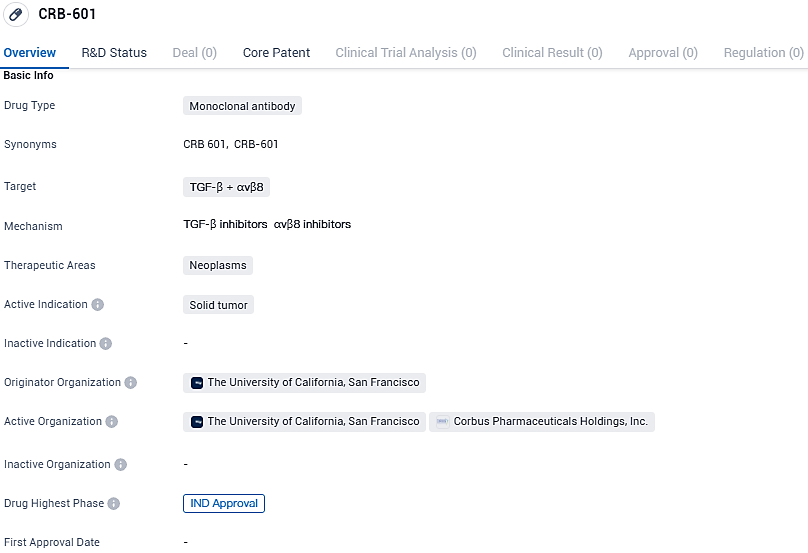
Corbus Pharma Secures US FDA Approval for Phase 1 Trial of its Novel αvβ8 Antibody, CRB-601
Corbus Pharmaceuticals Holdings, Inc., a company dedicated to precision cancer treatments and boasting a multifaceted product range, has revealed that the U.S. Food and Drug Administration has granted approval for their experimental drug filing for CRB-601. This drug, considered a leading candidate in its class, is a TGFβ inhibiting monoclonal antibody that specifically targets the integrin αvβ8.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
At the Society for Immunotherapy of Cancer's 38th Annual Meeting, researchers released preliminary data pointing out that CRB-601 successfully countered the mechanisms by which tumors evade immune detection while also potentiating the effectiveness of immune checkpoint inhibitors when tested in living organisms. It's the Company's ambition to start recruiting for the initial phase of the clinical trial in the earlier portion of 2024.
As the Chief Executive Officer of Corbus, Yuval Cohen, Ph.D., stated, “Recent experimental results indicate that CRB-601 exhibits significant anti-cancer properties by itself and when used with anti-PD-1 treatments across various solid tumor types with different levels of response to PD(L)-1 blockades.”
Further elaborating, “Our studies reveal that CRB-601 interferes with the release of active TGFβ and rewards the immune cells' invasion into the tumors at the cellular level in experimental settings. The anticipated synergistic benefit of this approach combined with anti PD(L)-1 interventions is promising. We're eager about moving ahead with the First-in-Human phase 1 trial for CRB-601 and foresee the beginning of participant recruitment before mid-year,” added Yuval Cohen, Ph.D.
Included in Corbus’ development pipeline are: CRB-701, a novel antibody-drug conjugate targeting Nectin-4 expression on malignancies to dispense a lethal payload; CRB-601, a tailored monoclonal antibody that hinders TGFβ activation found on tumor cells; and CRB-913, a potent CB1 inverse agonist confined mainly to the periphery, aimed at obesity therapy. Corbus is based out of Norwood, Massachusetts.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
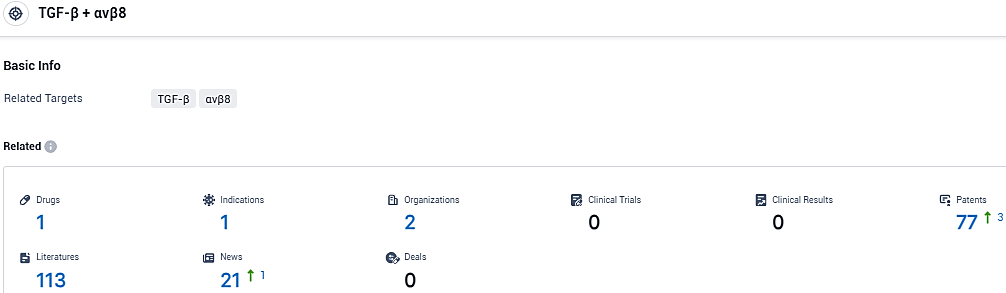
According to the data provided by the Synapse Database, As of January 15, 2024, there are 1 investigational drugs for the TGF-β and αvβ8 tagets, including 1 indications, 2 R&D institutions involved, and as many as 77 patents.
CRB-601 is a monoclonal antibody drug that targets TGF-β and αvβ8 for the treatment of neoplasms, specifically solid tumors. It is being developed by The University of California, San Francisco and has reached the highest phase of development, IND approval. This information provides a brief overview of the drug and its current status in the pharmaceutical industry.