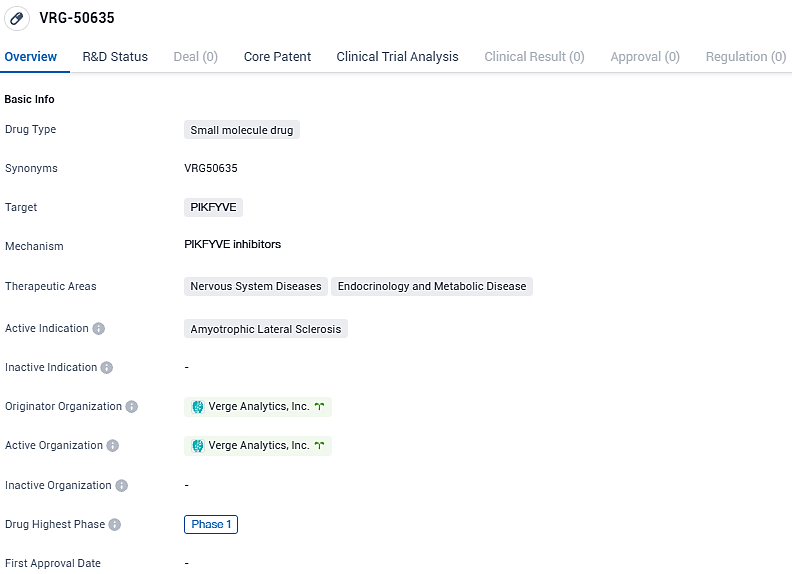
Verge Genomics has launched a preliminary study for VRG50635 as a potential ALS therapy
Verge Genomics, a biotech entity in the clinical phase dedicated to revolutionizing the process of discovering and developing drugs with the aid of artificial intelligence and real-world human data, has declared the commencement of its Phase 1b exploratory trial concerning VRG50635, which is being investigated for its potential therapeutic effects on both sporadic and familial types of amyotrophic lateral sclerosis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Our investigation will evaluate the effects and acceptability of increased dosage levels of VRG50635, a market-leading, compact molecule suppressor of PIKfyve, identified as a key therapeutic site for ALS within pathological human specimens through the utilization of CONVERGE®, our proprietary AI-driven, all-human diagnostic methodology. VRG50635 stands out as one of the pioneer medications debuted in the clinical sphere that has been fully identified and cultivated through an AI-sophisticated infrastructure.
In past ALS examinations, the evolution of an individual is gauged within a clinic through inferior, clinician-applied evaluative metrics, which often yield limited input critical to affirming the foundational theory in the novice phase of clinical exploration. In a departure from this, Verge's research employs contemporary digital apparatus to procure an extensive array of precise, measurable, significant data points straight from subjects, encompassing aspects like maneuverability, respiratory function, and sleep patterns, all within the convenience of their living spaces.
This caliber of information about the patient significantly augments our capacity to perceive the advancement of the disease and alterations due to treatments, thus enabling a tailored evaluation of variation for every participant as they progress from a non-medicated state to incrementally larger quantities of VRG50635 over extended durations of application.
"Launching this proof-of-concept examination signifies a pivotal achievement for Verge, reinforcing our ability to streamline the pursuit and refinement of potential treatments for life-threatening afflictions such as ALS," communicated Alice Zhang, the CEO and co-creator of Verge Genomics. "The prospective game-changing impact of our CONVERGE® platform has us very optimistic, as it promises to hasten the translation of laboratory research to patient care, ameliorating health outcomes globally."
"Verge deserves commendation for their innovative approach to proof-of-concept formulation, which integrates avant-garde technologies and novel scientific advancements in their mission to develop groundbreaking ALS therapies," remarked Dr. Angela Genge, Director at the ALS Centre of Excellence for Research and Patient Care housed within the Montreal Neurological Institute-Hospital.
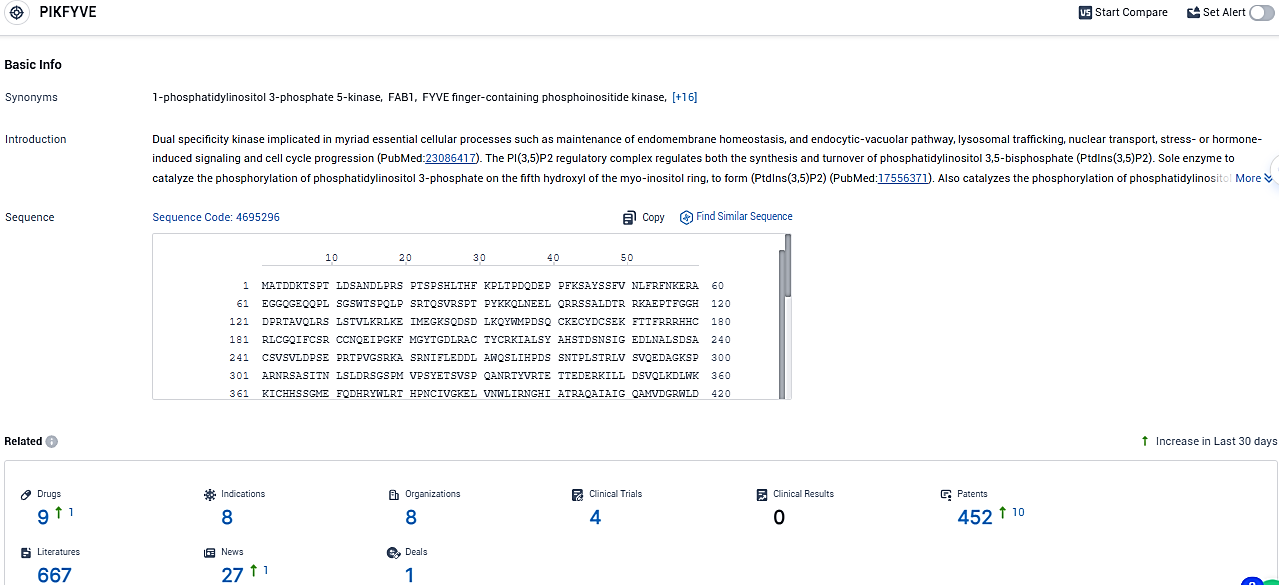
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 15, 2024, there are 9 investigational drugs for the PIKfyve target, including 8 indications, 8 R&D institutions involved, with related clinical trials reaching 4, and as many as 452 patents.
VRG50635 is a potent, orally bioavailable PIKfyve inhibitor that improves survival in amyotrophic lateral sclerosis patient neurons and has shown efficacy in multiple preclinical studies in ALS-relevant models of motor neuron degeneration. VRG50635 is the only PIKfyve inhibitor in clinical development that has been specifically optimized for the treatment of central nervous system disorders like ALS, with the potential to become a best-in-class therapy.