Stage III research on survodutide for individuals struggling with obesity and overweight, including those with or without diabetes, heart disease, and persistent kidney disorders
In conjunction with Zealand Pharma A/S, Boehringer Ingelheim has initiated three Phase III research investigations for survodutide (also known as BI 456906) for people with overweight or obesity issues. The formulation of these trials is based on the revelations from Phase II, where weight reductions of up to 19 percent were experienced by overweight or obese individuals. The recruitment process for these Phase III investigations will begin soon.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Expanded findings from the Phase II research, showcased at the 59th yearly conference of the European Association for the Study of Diabetes, illustrated declines in concrete waistline measurements, tangible body mass, and actual systolic and diastolic blood pressure over a period of 46 weeks.
"As obesity rates continue to surge, there's an urgent need for us to devise fresh and innovative strategies to grapple with this grave, persistent health condition," stated Carel le Roux, M.D., Ph.D., a professor at University College in Dublin, Ireland, and the project's lead researcher.
Carel le Roux, M.D. added, "Survodutide works in a unique way that could potentially decrease appetite while boosting liver energy consumption. This encouraging Phase II data make us optimistic about survodutide’s potential as a therapeutic strategy for individuals afflicted with obesity."
"By leveraging the meaningful learnings from the Phase II research, we are optimistic about the fast-paced development of survodutide," noted Carinne Brouillon, the lead of Human Pharma at Boehringer Ingelheim. "Through these trial initiations, we constantly capitalize on our legacy of introducing varied and groundbreaking therapies to combat cardiovascular, renal, and metabolic conditions."
"We're thrilled that survodutide will shortly proceed to Phase III trials as part of the worldwide SYNCHRONIZE program for individuals struggling with overweight or obesity," said David Kendall, MD, and the Chief Medical Officer for Zealand Pharma. "We are focusing on pivotal metabolic pathways, and this therapeutic approach could potentially tackle one of the most critical healthcare issues in contemporary medicine."
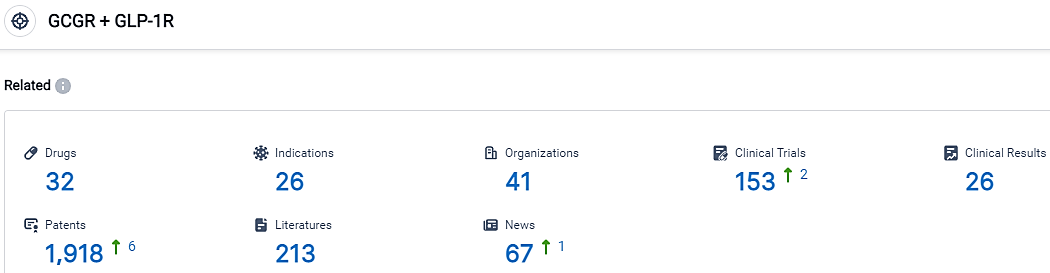
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of October 17, 2023, there are 32 investigational drugs for the GCGR and GLP-1R target, including 26 indications, 41 R&D institutions involved, with related clinical trials reaching 153,and as many as 1918 patents.
Survodutide operates as a dual agonist for the glucagon/GLP-1 receptor, triggering both the GLP-1 and glucagon receptors that are integral to the regulation of metabolic activities. It is projected that the trial will be finalized in the 4th quarter of 2023. The U.S. FDA has granted Survodutide an expedited review process, also known as a Fast Track designation, for use in adults diagnosed with NASH.