Taurolidine Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Taurolidine's R&D Progress
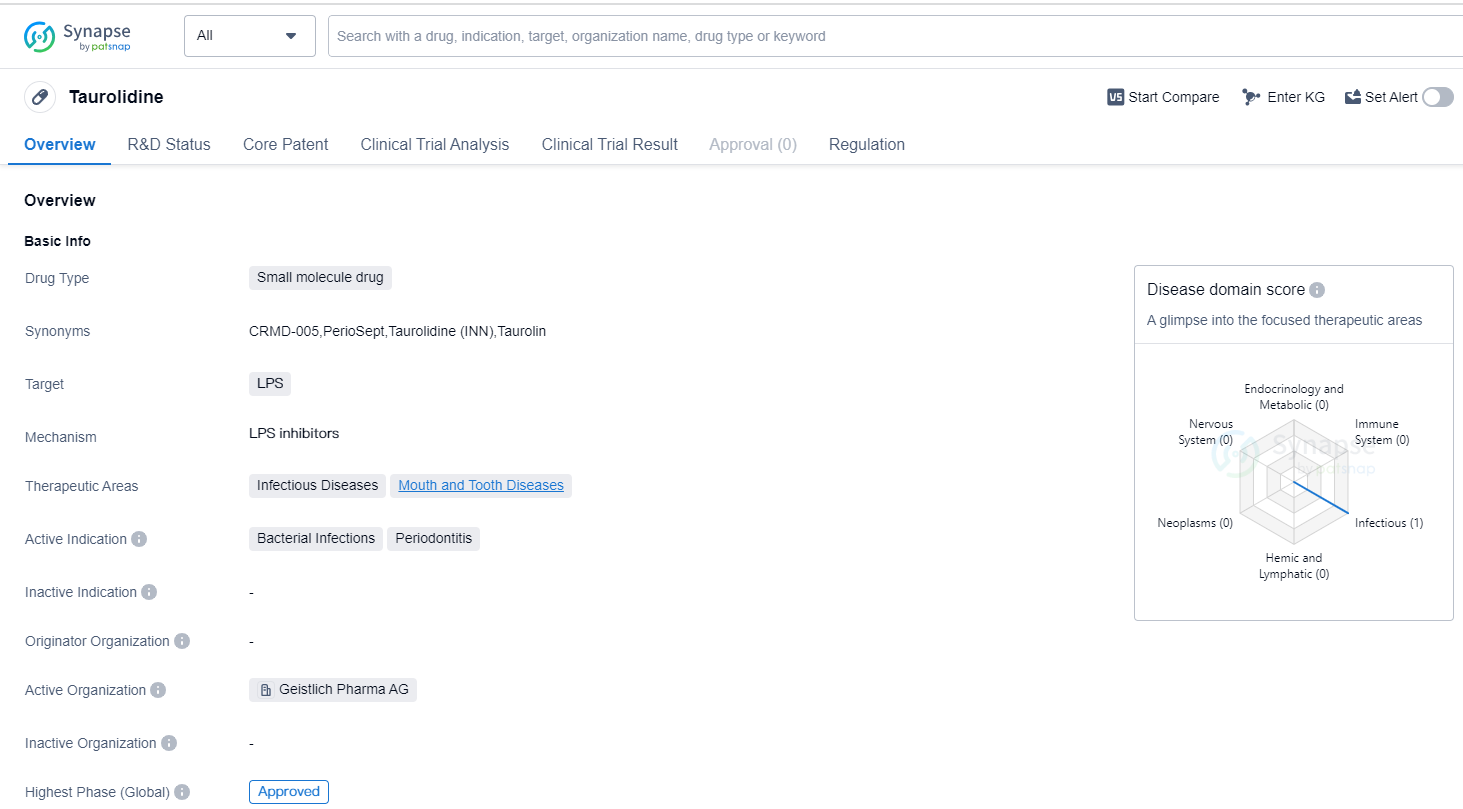
Taurolidine is a small molecule drug that primarily targets lipopolysaccharides (LPS). It is used in the treatment of infectious diseases and mouth and tooth diseases. The drug is specifically indicated for bacterial infections and periodontitis.
The highest R&D phase of this drug is approved. This means that it has successfully completed clinical trials and has been granted regulatory approval for use in patients. This indicates that the drug has demonstrated safety and efficacy in treating the targeted conditions.
In terms of regulation, Taurolidine is classified as an orphan drug. Orphan drugs are medications that are developed to treat rare diseases or conditions that affect a small number of patients. The designation of orphan drug provides certain incentives to pharmaceutical companies, such as extended market exclusivity and financial support, to encourage the development of treatments for these rare conditions.
Based on the available information, Taurolidine appears to be a promising drug in the field of biomedicine. Its small molecule nature allows for efficient delivery and potential targeting of LPS, which is a key component of bacterial infections. The drug's therapeutic areas of focus, infectious diseases and mouth and tooth diseases, indicate its potential to address a wide range of conditions.
The approval of Taurolidine suggests that it has undergone rigorous testing and has met the necessary regulatory requirements for safety and efficacy. This is a positive indication of its potential as a treatment option for bacterial infections and periodontitis.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for taurolidine: LPS inhibitors
LPS inhibitors are substances or compounds that can inhibit the activity or production of lipopolysaccharide (LPS). LPS is a molecule found on the outer membrane of certain bacteria, such as Gram-negative bacteria. It plays a crucial role in the pathogenesis of bacterial infections and can trigger an immune response in the host.
From a biomedical perspective, LPS inhibitors are of great interest in the field of infectious diseases and immunology. By inhibiting LPS, these compounds can potentially prevent or reduce the harmful effects of bacterial infections. LPS inhibitors can target various stages of LPS biosynthesis or its interaction with host cells, thereby interfering with the bacteria's ability to cause infection.
Inhibition of LPS can have several therapeutic benefits. It can help in reducing the release of pro-inflammatory cytokines, which are responsible for the symptoms associated with bacterial infections, such as fever and inflammation. LPS inhibitors can also prevent the activation of immune cells and the subsequent cascade of inflammatory responses, leading to a better control of the infection.
Furthermore, LPS inhibitors may have potential applications in the development of vaccines or adjuvants. By blocking LPS, they can reduce the toxicity of certain vaccines that contain LPS as a component. This can improve the safety profile of vaccines and enhance their effectiveness.
Overall, LPS inhibitors are important tools in the fight against bacterial infections and have the potential to contribute to the development of novel therapeutic strategies.
Drug Target R&D Trends for taurolidine
Lipopolysaccharides (LPS) are large molecules found in the outer membrane of certain bacteria, such as gram-negative bacteria. In the human body, LPS plays a crucial role in the immune response. When bacteria invade the body, LPS acts as a potent stimulator of the immune system, triggering the release of pro-inflammatory cytokines and activating immune cells. However, excessive or uncontrolled activation of LPS can lead to harmful effects, such as sepsis or chronic inflammation. Understanding the role of LPS in the human body is essential for developing strategies to modulate its effects and potentially treat or prevent associated diseases.
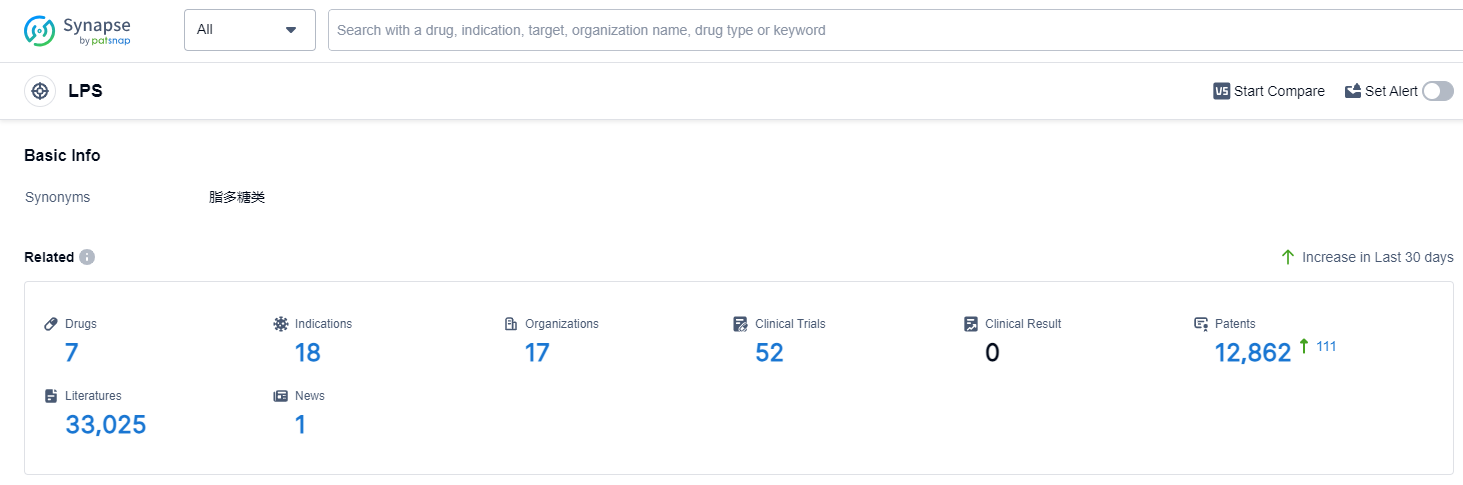
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 7 LPS drugs worldwide, from 17 organizations, covering 18 indications, and conducting 52 clinical trials.
Overall, the target LPS presents a competitive landscape with promising developments in the pharmaceutical industry. The future development of drugs targeting LPS holds great potential for addressing various medical conditions and improving patient outcomes.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Taurolidine's status as an approved drug and its orphan drug designation highlight its potential value in the pharmaceutical industry. Further research and development may uncover additional therapeutic applications for this small molecule drug, potentially expanding its impact in the field of biomedicine.