Stallerges Greer Announces Global Nestle Contract for Palforzia® Peanut Allergy Treatment
Stallergenes Greer, a top-tier global healthcare enterprise focusing on allergen immunotherapy, revealed today that it has inked global contracts with Nestlé to exploit the oral immunotherapy solution for peanut allergy, Palforzia®. The deal was sealed at the time of signing.
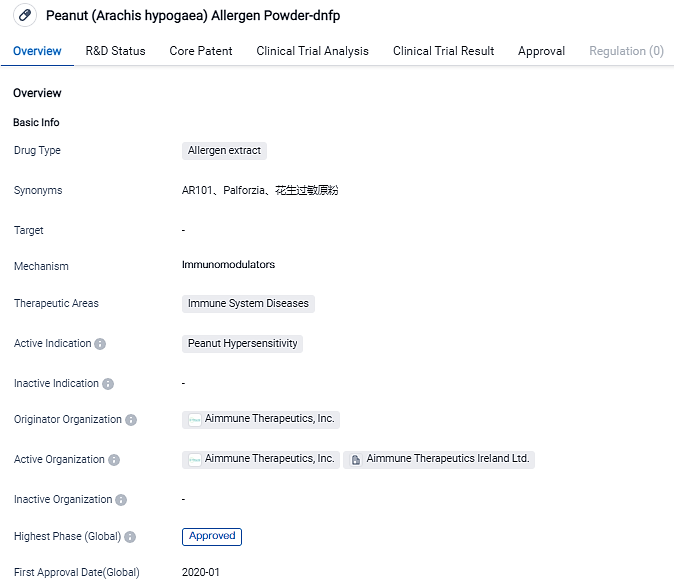
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Peanut allergy is a condition that currently impacts about 2% of the population in Western countries. From 2005 to 2015, there was a two-fold increase in the occurrence of this allergy amongst children. The challenge lies in preventing contact with peanuts, given the acute nature of the allergic responses, which may include anaphylaxis. This underscores the necessity for competent treatment protocols.
"We are thrilled about this contract with Nestlé, as it signifies a substantial achievement for Stallergenes Greer," announced Michele Antonelli, CEO of Stallergenes Greer. In terms of combating accidental exposure to peanuts, Palforzia®, boasting a strong effectiveness and safety track record, is presently the sole endorsed treatment in use.
Initiating their incursion into the food allergy sector, Stallergenes Greer has established itself as the premier allergen immunotherapy firm delivering both respiratory and food allergy solutions. This reflects the enduring pledge of the company to offer cutting-edge allergen immunotherapy treatments to both patients and the healthcare community.
Stallergenes Greer is set to make milestone payouts and continuing interest payments to Nestlé. A standard transition phase will take place to sustain business stability and ensure patients have unimpeded treatment access.
Through this business deal, Stallergenes Greer solidifies its position in the AIT domain. The company’s collection of treatments provides both patients and medical experts with accurate, customizable AIT therapies that cater to a wide variety of administration methods. These can be specifically adapted to cater to the unique requirements of allergy sufferers, including oral sublingual solutions, tablets, injectables for breathing allergies, and powder for mixing with semi-solid food in the case of peanut allergies.
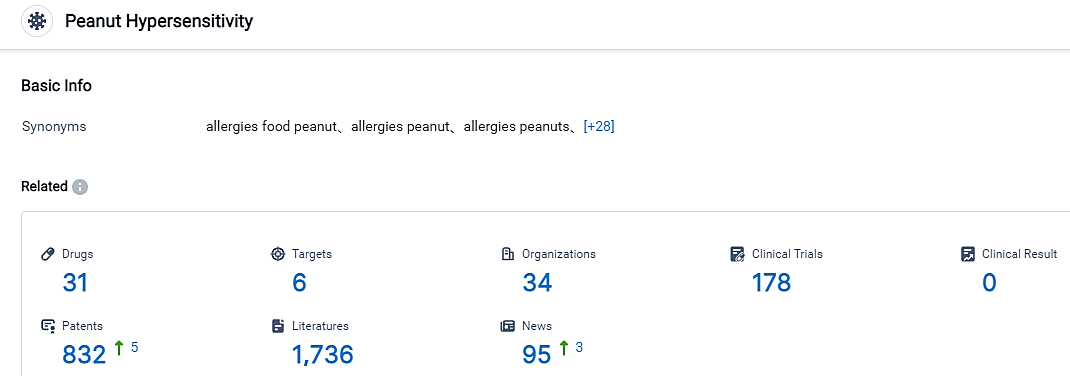
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of September 5, 2023, there are 31 investigational drugs for the Peanut hypersensitivity, including 6 targets, 34 R&D institutions involved, with related clinical trials reaching 178, and as many as 832 patents. The U.S. Food and Drug Administration (FDA), the European Commission (EC), the Medicines and Healthcare products Regulatory Agency (MHRA) in the U.K., and Swissmedic have granted approval to use Palforzia® as a form of oral immunotherapy to lessen the severity of allergic responses, including anaphylaxis, which may arise from unintentional exposure to peanuts. This treatment can be utilized by patients aged 4 to 17, who have been medically diagnosed with a peanut allergy. Continuing use of Palforzia® into adulthood, starting from 18 years onwards, is permissible. However, it's important to note that Palforzia® isn't a solution for immediate relief from allergic reactions or anaphylaxis, and should be administered in combination with a diet avoiding peanuts.