Star Therapeutics Shares Midpoint Results for VGA039 in VWD at 2024 ASH
Clinical-stage biotech firm Star Therapeutics, which specializes in developing top-tier antibodies, has released preliminary clinical findings from the VIVID 2 trial (VGA039 Investigation in Von Willebrand Disease). This Phase 1 single ascending dose (SAD) study examines VGA039, an experimental monoclonal antibody designed to target Protein S, thereby normalizing blood clotting as a universal hemostatic treatment for various bleeding disorders. VGA039 could be the first subcutaneous treatment effective against all VWD types and offers a user-friendly dosing schedule. The VGA039 clinical results are being showcased at the American Society of Hematology (ASH) annual meeting, held from December 7-10, in San Diego, California.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
Preliminary clinical findings from the VIVID 2 study involving three VWD patients with elevated bleeding rates indicated that a subcutaneous administration of VGA039 led to significant decreases in annualized bleed rate (ABR), comparable to existing VWD prophylactic treatments. The interim data from VIVID 2 revealed that VGA039 maintained therapeutic levels for several weeks after a single subcutaneous injection, unlike current VWD prophylaxis therapies that necessitate frequent weekly intravenous (IV) infusions. These VWD patients experienced high bleeding rates with ABRs exceeding 50 incidents annually.
"Advancements in VWD treatments have not kept pace with those for other bleeding disorders, such as hemophilia. VGA039 represents a potential breakthrough as a subcutaneous treatment, which could revolutionize VWD care by alleviating the treatment burden," remarked Dr. Nicholetta Machin from the Hemophilia Center of Western Pennsylvania and the University of Pittsburgh. "The interim clinical data is especially promising, demonstrating a substantial reduction in bleeding in VWD patients with high initial bleeding rates.”
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
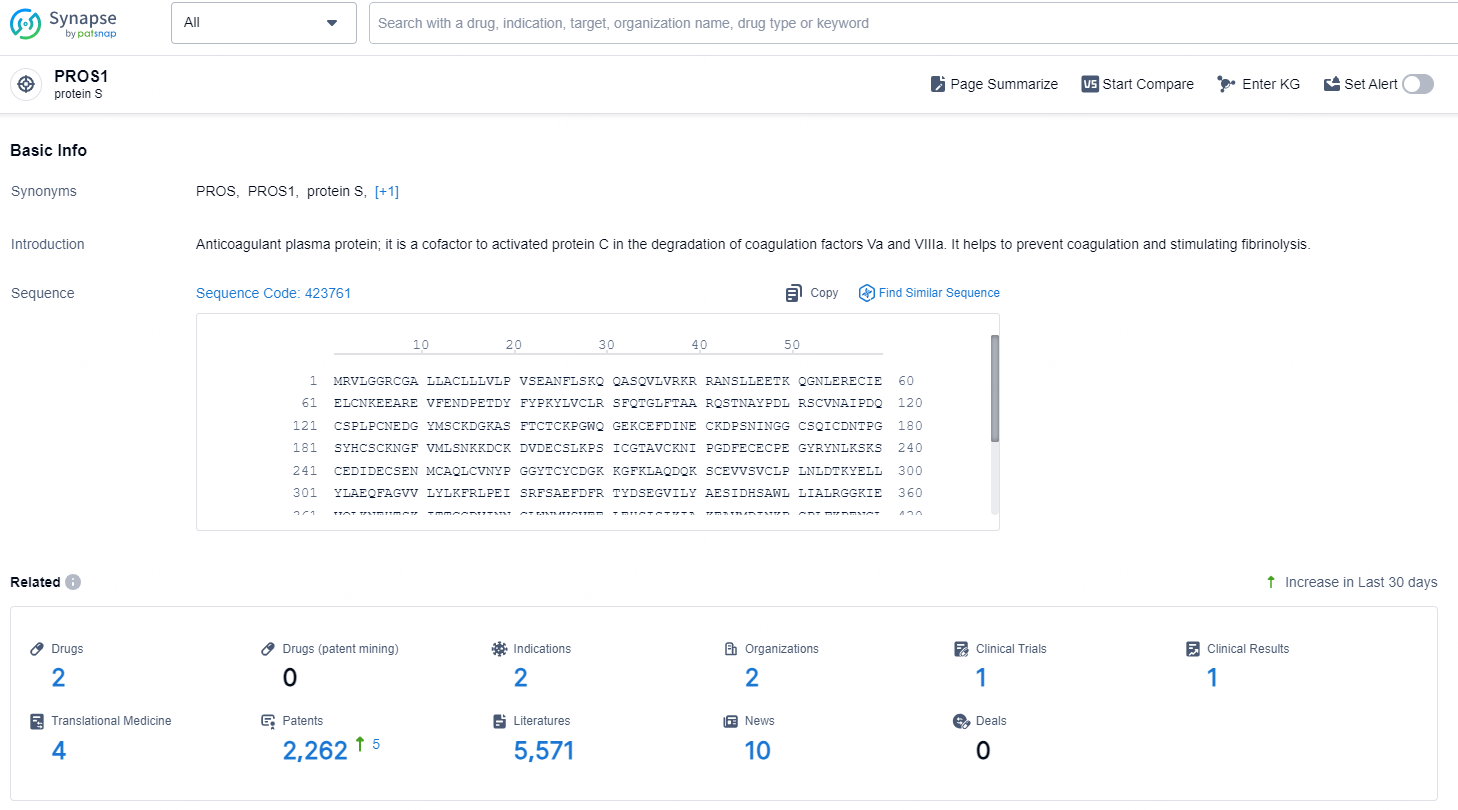
According to the data provided by the Synapse Database, As of December 13, 2024, there are 2 investigational drugs for the PROS1 target, including 2 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 2262 patents.
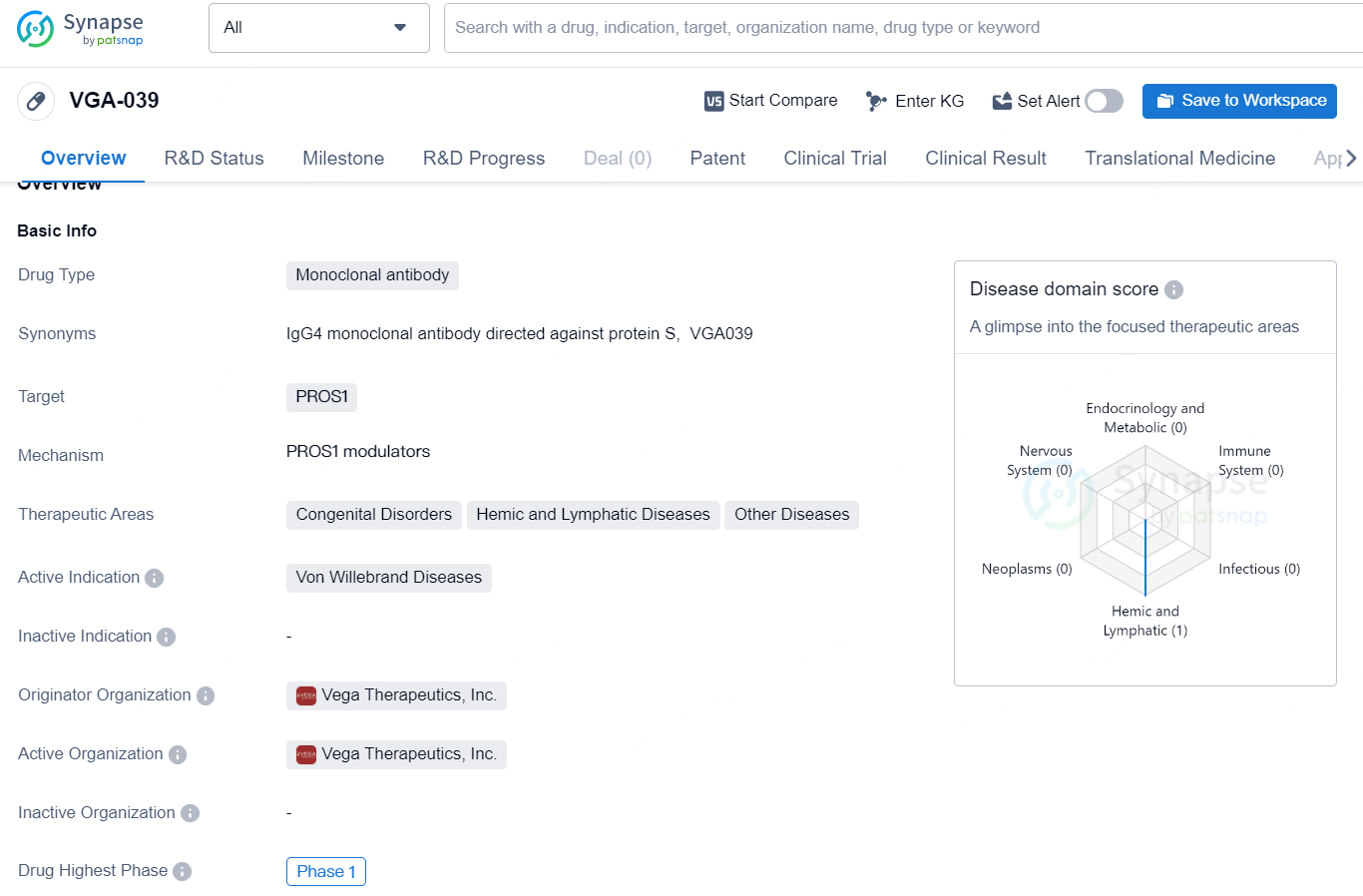
VGA-039 is a monoclonal antibody drug that targets PROS1 and is developed by Vega Therapeutics, Inc. The drug falls under the category of Orphan Drugs, indicating that it is intended to treat rare medical conditions. Its primary therapeutic areas are Congenital Disorders, Hemic and Lymphatic Diseases, and Other Diseases, with a specific active indication for Von Willebrand Diseases. As of the latest available data, VGA-039 has reached the highest global development phase of Phase 1.