Takeda Unveils Promising Phase 2b Mezagitamab Data for Primary Immune Thrombocytopenia Treatment
Takeda revealed encouraging outcomes from its Phase 2b trial, which was randomized, double-blind, and placebo-controlled. The study assessed the safety, tolerability, and effectiveness of mezagitamab (TAK-079) in individuals suffering from persistent or chronic primary immune thrombocytopenia, a rare disorder caused by the immune system that leads to bleeding. ITP is marked by the rapid destruction of blood platelets, leading to a reduced platelet count and exacerbating bleeding, which can be severely disabling.
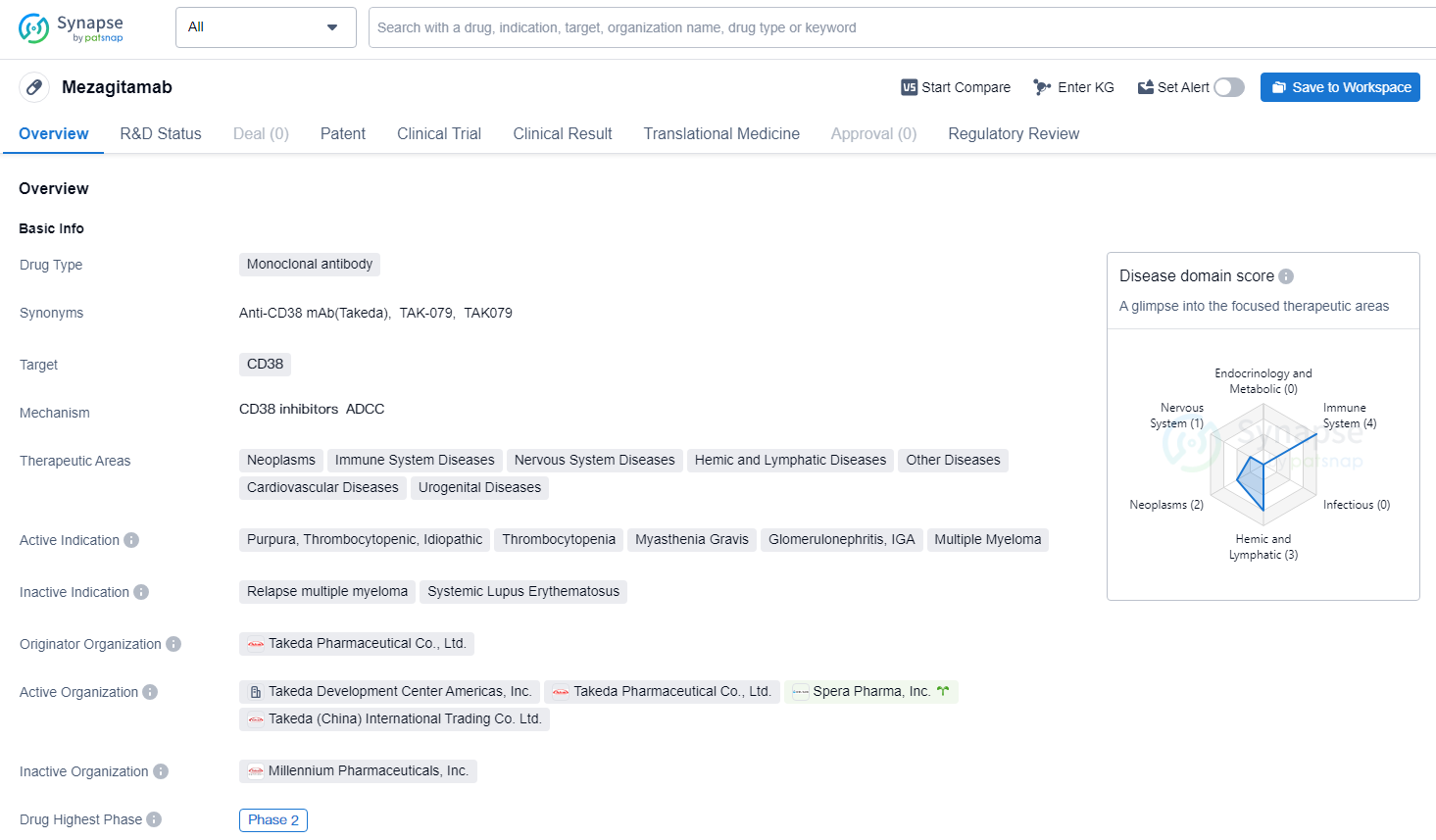
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
These findings were disclosed during the oral Late-Breakthrough Session at the 32nd Congress of the International Society on Thrombosis and Haemostasis, held in Bangkok, Thailand. Takeda intends to begin a global Phase 3 study of mezagitamab for patients with ITP in the latter half of FY2024.
The TAK-079-1004 study assessed three different subcutaneous doses of mezagitamab versus placebo, administered once a week for eight weeks to individuals with chronic or persistent primary ITP, followed by more than eight weeks of safety monitoring. The main endpoint is the proportion of patients who experience at least one Grade 3 or higher treatment-emergent adverse event, serious adverse event, or adverse event leading to the cessation of mezagitamab. Secondary endpoints include platelet response, complete platelet response, clinically meaningful platelet response, and hemostatic platelet response.
Results from the Phase 2b trial indicated that mezagitamab treatment improved platelet response in comparison to placebo at all three dosage levels tested. Patients receiving mezagitamab experienced rapid and sustained increases in platelet counts, persisting for eight weeks post-final dose through to Week 16, showcasing the swift and lasting effects of mezagitamab on platelet response.
"We are honored to have these Phase 2b mezagitamab findings selected for a late-breaking abstract presentation at the ISTH Congress," stated Obi Umeh, M.D., M.Sc., Vice President, Franchise Global Program Leader at Takeda. "Based on these outcomes, we aim to initiate a Phase 3 study of mezagitamab in ITP in the latter half of FY2024, further underscoring our commitment to developing groundbreaking treatments in therapeutic areas with significant unmet patient needs."
Mezagitamab is a fully human IgG1 monoclonal antibody that binds with high affinity to CD38-expressing cells, leading to their depletion. The aim of therapy with mezagitamab is to provide rapid and sustained improvement in platelet response and to restore platelet counts to functional levels.
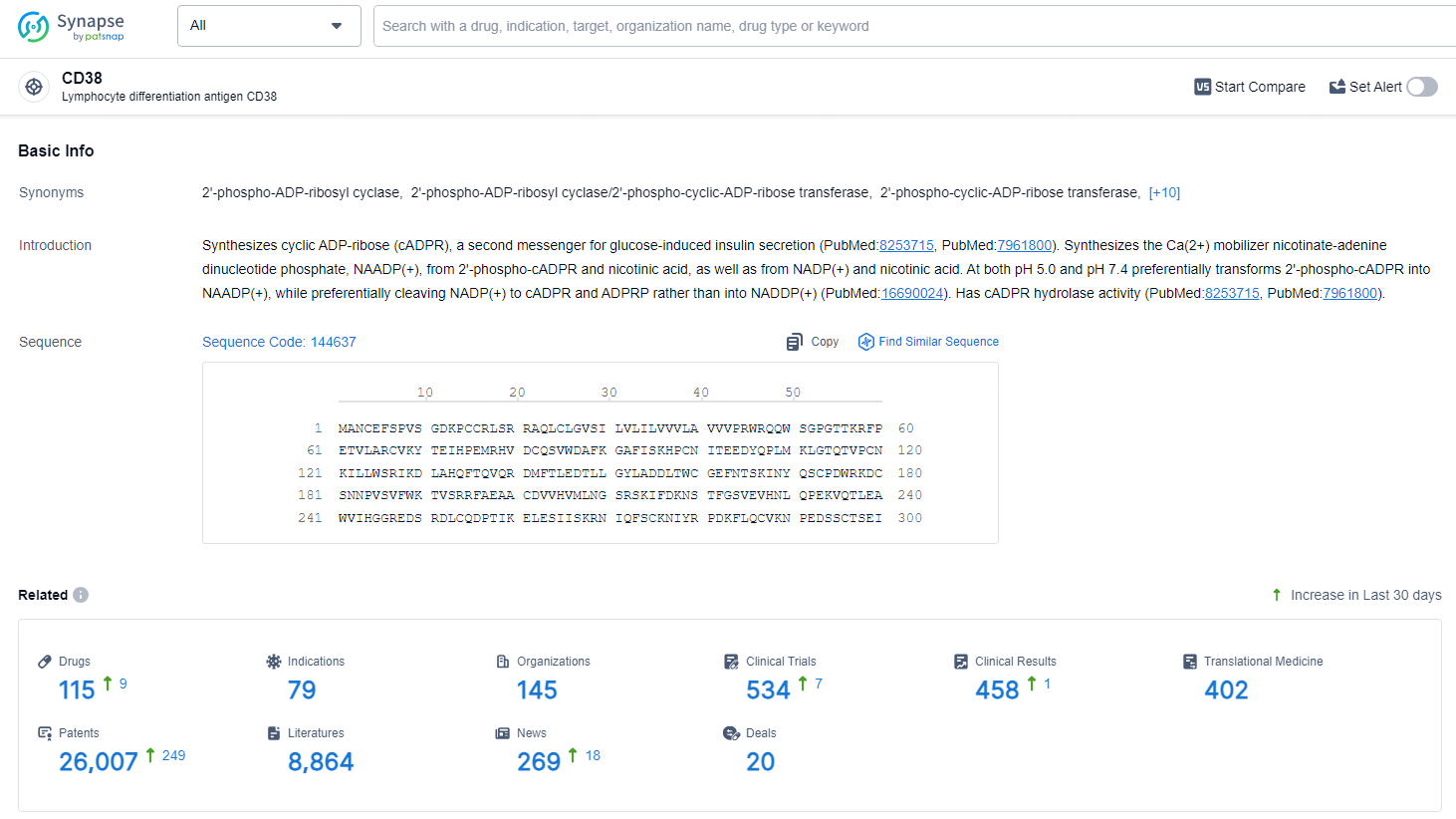
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of June 27, 2024, there are 115 investigational drugs for the CD38 target, including 79 indications, 145 R&D institutions involved, with related clinical trials reaching 534, and as many as 26007 patents.
Mezagitamab previously received Orphan Drug Designation for the treatment of ITP and Fast Track Designation for treatment of chronic/persistent ITP from the U.S. Food and Drug Administration. Mezagitamab is an investigational compound that has not been approved for use by any regulatory authority.