Suzetrigine: FDA-Approved Nav1.8 Inhibitor Offers Breakthrough Non-Opioid Relief for Moderate to Severe Acute Pain
Suzetrigine received approval from the U.S. Food and Drug Administration (FDA) on January 30, 2025, as a small-molecule pharmaceutical for the treatment of moderate to severe acute pain in adults.
Mechanism of Action
Voltage-gated sodium channel Nav1.8 is a specialized sodium ion channel that plays a crucial role in the nervous system. Nav1.8 is primarily expressed in sensory neurons of the peripheral nervous system (PNS) and exhibits relative resistance to tetrodotoxin (TTX). This channel is particularly important for the transmission of pain signals, as it facilitates the generation and propagation of action potentials in response to noxious stimuli, especially in chronic pain conditions. Due to its critical role in pain transmission, Nav1.8 has emerged as a potential therapeutic target for the development of novel analgesic drugs.
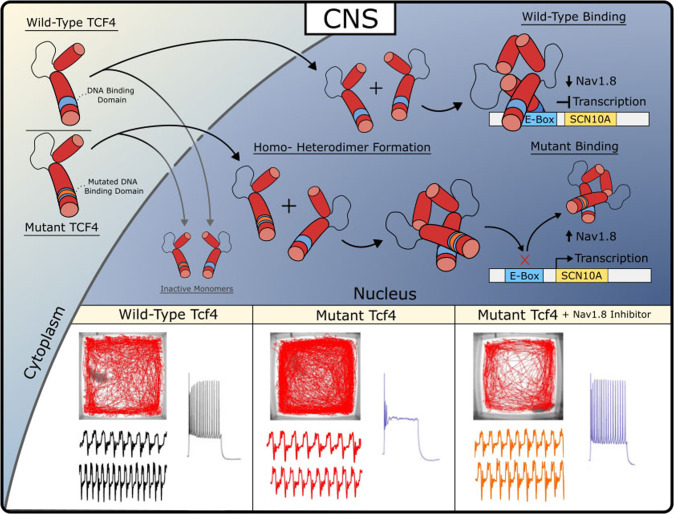
Suzetrigine acts as an X-type sodium channel α-subunit blocker, selectively inhibiting the Nav1.8 channel to prevent pain signal transmission. This high level of selectivity allows Suzetrigine to effectively alleviate pain while minimizing off-target effects on other sodium channels, thereby reducing the risk of side effects. Unlike opioids, Suzetrigine's mechanism of action avoids common opioid-related adverse effects such as addiction, respiratory depression, and constipation. This is because Suzetrigine specifically targets sodium channels that are predominantly involved in peripheral nociceptive neurons, rather than opioid receptors in the central nervous system.
For patients requiring non-opioid pain relief, Suzetrigine offers a significant new option. In the United States, more than 80 million people require pharmacological management for moderate to severe acute pain each year, highlighting the urgent need for effective, non-addictive analgesics. The successful development and approval of Suzetrigine mark the first market entry of a novel pain treatment mechanism in nearly two decades. Clinical trials conducted in patients undergoing abdominoplasty and bunionectomy demonstrated that Suzetrigine significantly reduced pain scores without exhibiting addiction potential.
How to obtain the latest research advancements in the field of biopharmaceuticals?
In the Synapse database, you can keep abreast of the latest research and development advances in drugs, targets, indications, organizations, etc., anywhere and anytime, on a daily or weekly basis. Click on the image below to embark on a brand new journey of drug discovery!
Reference:
- 1. Martinowich K, Das D, Sripathy SR, Mai Y, Kenney RF, Maher BJ. Evaluation of Nav1.8 as a therapeutic target for Pitt Hopkins Syndrome. Mol Psychiatry. 2023 Jan;28(1):76-82. doi: 10.1038/s41380-022-01811-4. Epub 2022 Oct 12. PMID: 36224259; PMCID: PMC9812766.