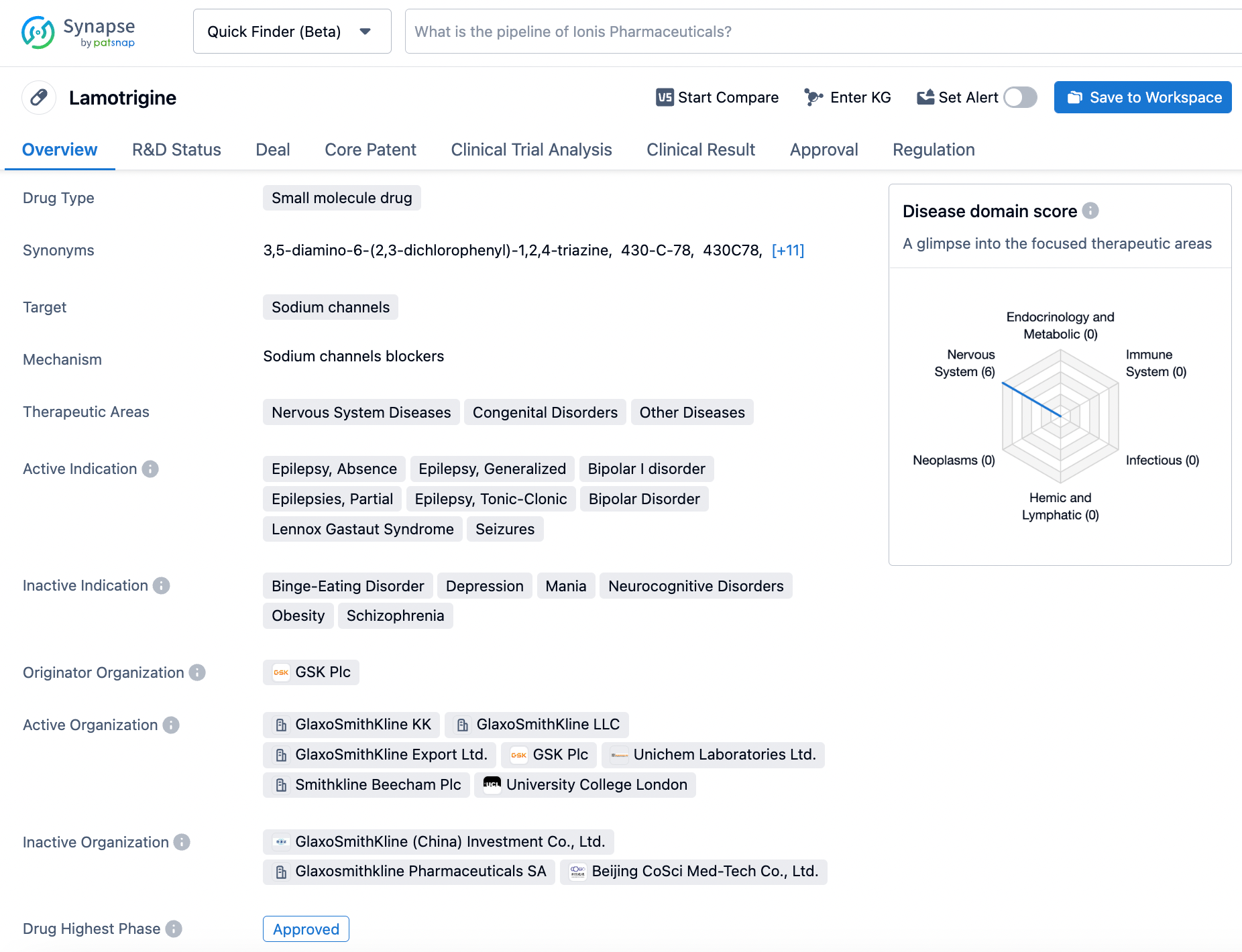
Synapse Simplified: How to Find Lamotrigine Information
Lamotrigine, a pharmacological agent granted approval by the United States Food and Drug Administration (FDA) on the 27th of December, 1994, is a preeminent anticonvulsant compound forged by the pharmaceutical giant GlaxoSmithKline. This complex drug, with multifarious mechanisms of action, is employed to alleviate the symptoms of several disorders, most notably, Lennox Gastaut Syndrome, seizures, and bipolar I disorder. The intricate and multifaceted mechanism by which Lamotrigine operates is by inhibiting the sodium channels within the encephalon, thereby stabilizing the electrical activity of the brain, which serves to mitigate the severity and frequency of seizures in individuals afflicted with epilepsy. Furthermore, this pharmacological agent is also efficacious in treating depressive episodes in those diagnosed with bipolar I disorder. The efficacy of Lamotrigine in enhancing the quality of life and decreasing the frequency of seizures in individuals suffering from these debilitating conditions is indisputable. Click on the image below to begin the exploration journey of Lamotrigine through the Synapse database!
You can search for the latest pharmaceutical information such as drugs, targets, patents, transactions, clinical results, etc. through the Synapse database. Come and experience it!