Synthekine's STK-012 Shows Promise in Treating Advanced Solid Cancers
Synthekine Inc., a company specialized in creating modified cytokine treatments, recently unveiled encouraging preliminary findings from an early-stage human study, specifically a Phase 1a/1b trial, focusing on their specialized drug STK-012. This drug is an α/β selective IL-2 subagonist that's being tested as a potential therapy for patients suffering from sophisticated solid cancers. The revealing of this research took place at the prominent American Association for Cancer Research's Annual Meeting in 2024, which was hosted in San Diego.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
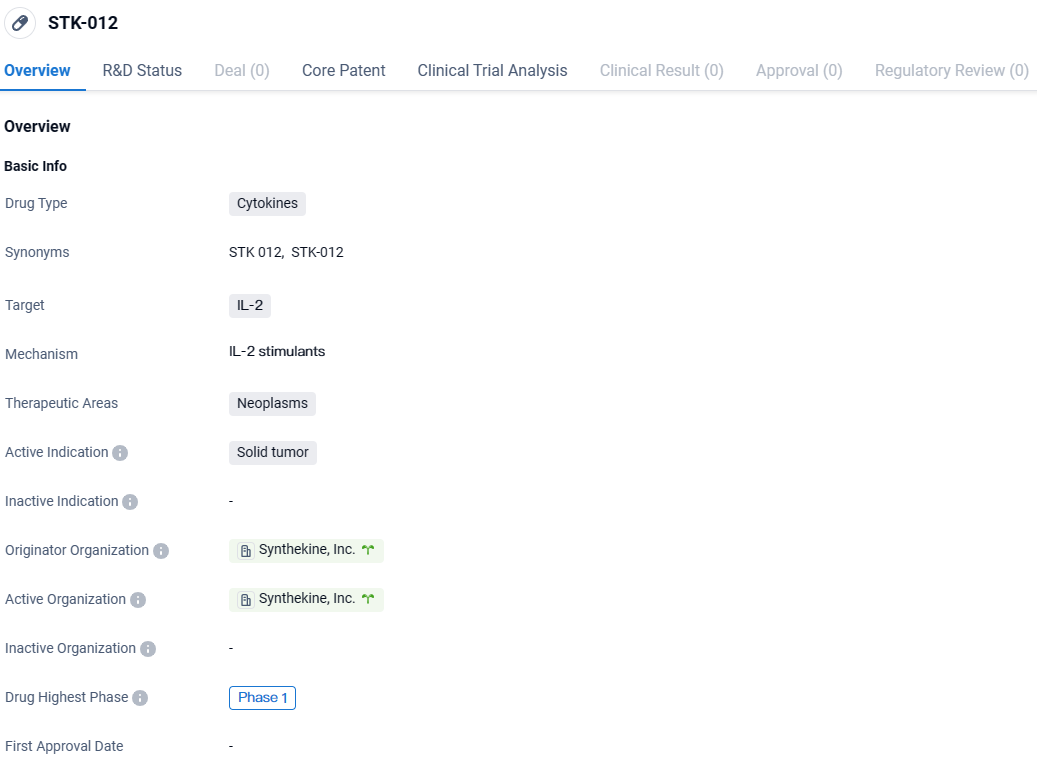
STK-012 stands out as a novel α/β-IL-2R biased partial agonist, tailor-made to selectively activate T cells that express CD25 and have been activated by antigens. These T cells play a crucial role in powerful anti-cancer responses. This agent specifically targets to boost these cells while minimizing the activation of other immune cells, like natural killer cells, which are linked to adverse effects stemming from IL-2. From the disclosed findings, encompassing treatment of 47 individuals in the initial dose-escalation stage of Phase 1a, STK-012 as a sole treatment showcased promising safety and effectiveness, as well as favorable pharmacokinetic and pharmacodynamic characteristics.
Naiyer Rizvi, M.D., the chief medical officer at Synthekine, reported, "The potential benefits of IL-2 for treating solid malignancies have not been fully harnessed, as prior versions of IL-2 analogs showed insufficient results and considerable toxicity levels."
Dr. Rizvi expressed enthusiasm: "With the use of STK-012 as a sole therapy, we're seeing a number of objective responses, propelled by the strong activation of interferon‐gamma (IFNγ) and the selective proliferation of antigen-stimulated T cells. Moreover, we are not observing the intense adverse effects that are commonly linked with IL-2 therapy, like low blood pressure, capillary leak syndrome, or liver enzyme elevations. We are eager to proceed with the dose expansion phase of our investigation to delve deeper into its prospects for patient care." Dr. Rizvi further stated.
Following the successful conclusion of the Phase 1a dose-escalation segment, Synthekine commenced the Phase 1b stage of its research in September 2023. The ongoing Phase 1b part includes an extension of dosage to gauge STK-012's solo efficacy at the suggested dose for Phase 2 in particular types of solid tumors, such as renal cell carcinoma and non-small cell lung cancer.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
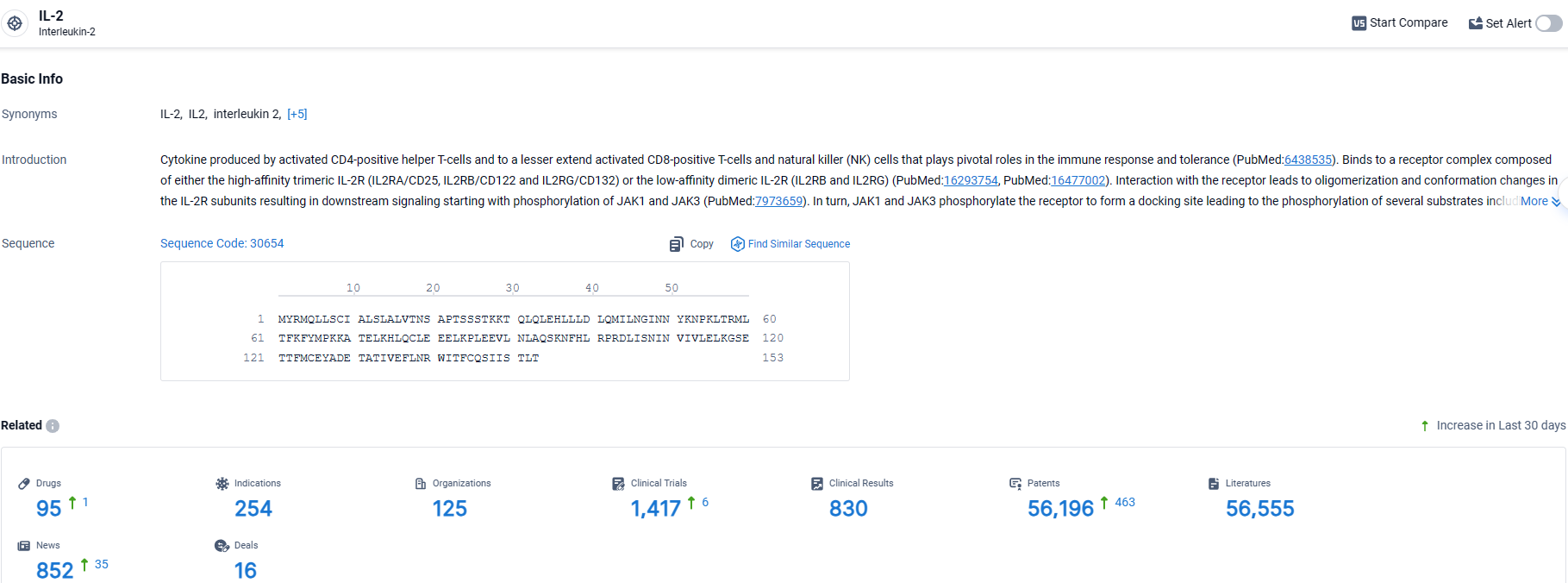
According to the data provided by the Synapse Database, As of April 10, 2024, there are 95 investigational drugs for the IL-2 target, including 254 indications, 125 R&D institutions involved, with related clinical trials reaching 1417, and as many as 56196 patents.
STK-012 is a cytokine drug being developed by Synthekine, Inc. It targets IL-2 and is intended for the treatment of solid tumors. The drug is currently in Phase 1 of development, indicating that it is still undergoing early clinical trials to assess its safety and dosage range. Further research and development will be necessary to determine the potential of STK-012 as a treatment option for solid tumors.