Pheast's Latest Preclinical Data on Anti-CD24 Inhibitor PHST001 Shared at SITC 2024
Pheast Therapeutics (“Pheast”), a biotechnology company focused on creating innovative macrophage checkpoint therapies to combat cancer, has revealed new preclinical findings for PHST001. This candidate, an anti-CD24 antibody, aims to obstruct a crucial “don’t eat me” signal on cancer cells that affects macrophages. These findings were shared at the 39th Annual Meeting of the Society for Immunotherapy of Cancer (SITC), held both online and in person at the George R. Brown Convention Center in Houston from November 6 to 10, 2024.
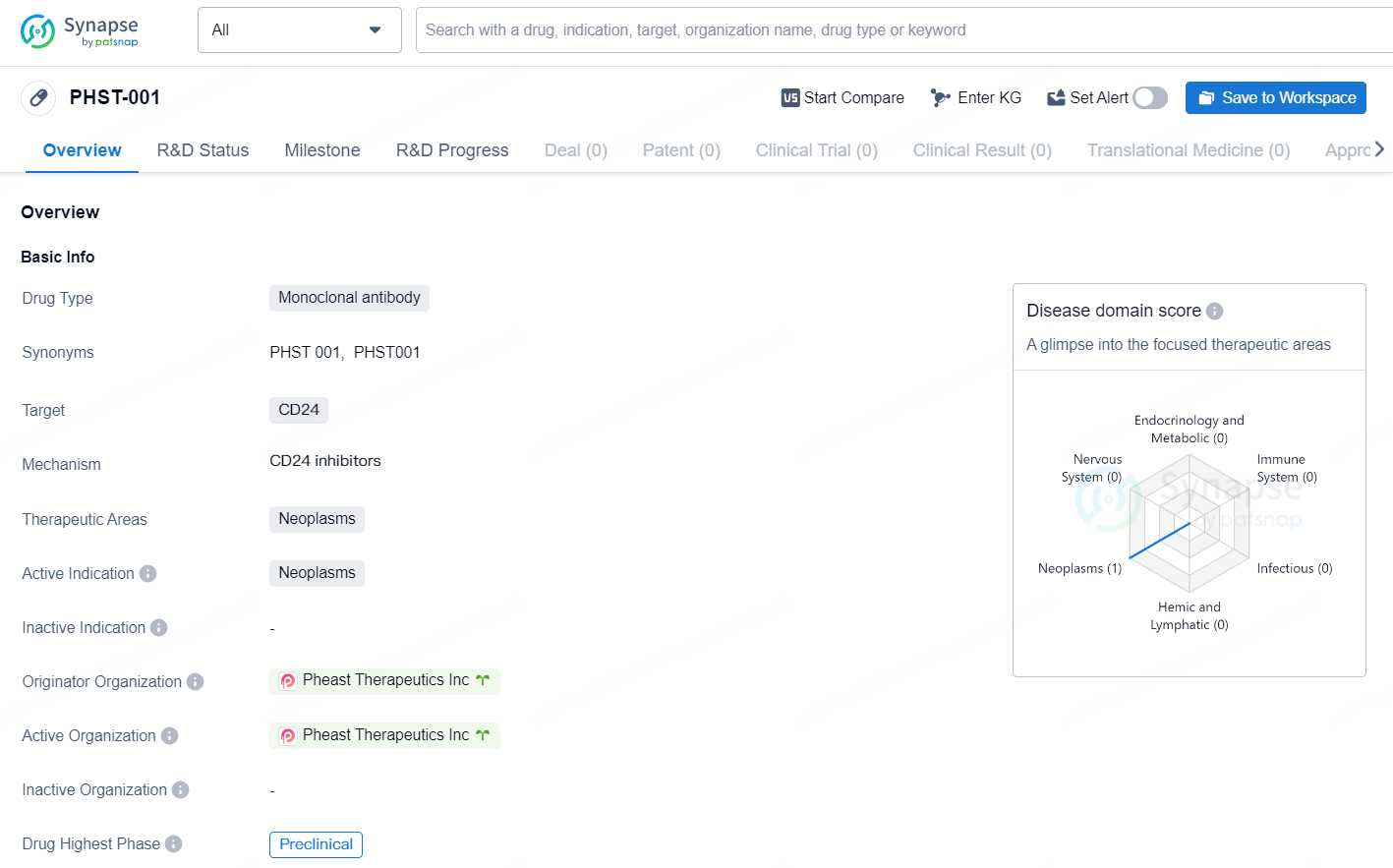
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
The data shared indicate that PHST001 enhances macrophage-mediated phagocytosis across various cancer cell types by effectively binding to CD24, leading to substantial tumor reduction in in vivo models. Furthermore, PHST001 exhibits a favorable pharmacokinetic profile in non-human primate studies and does not cause immune-mediated toxicity in vitro.
“These findings reinforce the outcomes we disclosed earlier this year at PEGS, where we demonstrated PHST001's strong ability to stimulate an anti-cancer immune response and enhance therapeutic effectiveness in complex mouse models,” stated Roy Maute, Ph.D., Cofounder and CEO of Pheast Therapeutics. “Macrophage checkpoint therapies like PHST001 could significantly broaden treatment possibilities for patients in oncology fields with substantial unmet needs, particularly where existing immunotherapies have fallen short.”
CD24 is notably overexpressed in various human cancers, such as ovarian cancer and triple-negative breast cancer (TNBC), and its elevated levels correlate with poor prognosis across multiple cancer types. CD24 interacts with the macrophage receptor Siglec-10, which protects cancer cells from macrophage-mediated destruction. Pheast has developed PHST001 to attach to CD24 present on cancer cell membranes with high specificity and affinity while obstructing Siglec-10 binding.
“The preclinical findings indicate that PHST001 has the potential to target several cancer types and is distinct as a new macrophage checkpoint inhibitor with strong CD24 inhibition,” indicated Suzana Kahn, Ph.D., Senior Director of Biology at Pheast Therapeutics. “With its non-toxic preclinical profile, alongside excellent efficacy in our preclinical studies, we are well positioned to commence first-in-human clinical trials.”
Dr. Kahn showcased this data in a poster presentation titled, “PHST001, a humanized anti-CD24 antibody, promotes phagocytosis of human tumor cells in vitro and facilitates tumor clearance in vivo.”
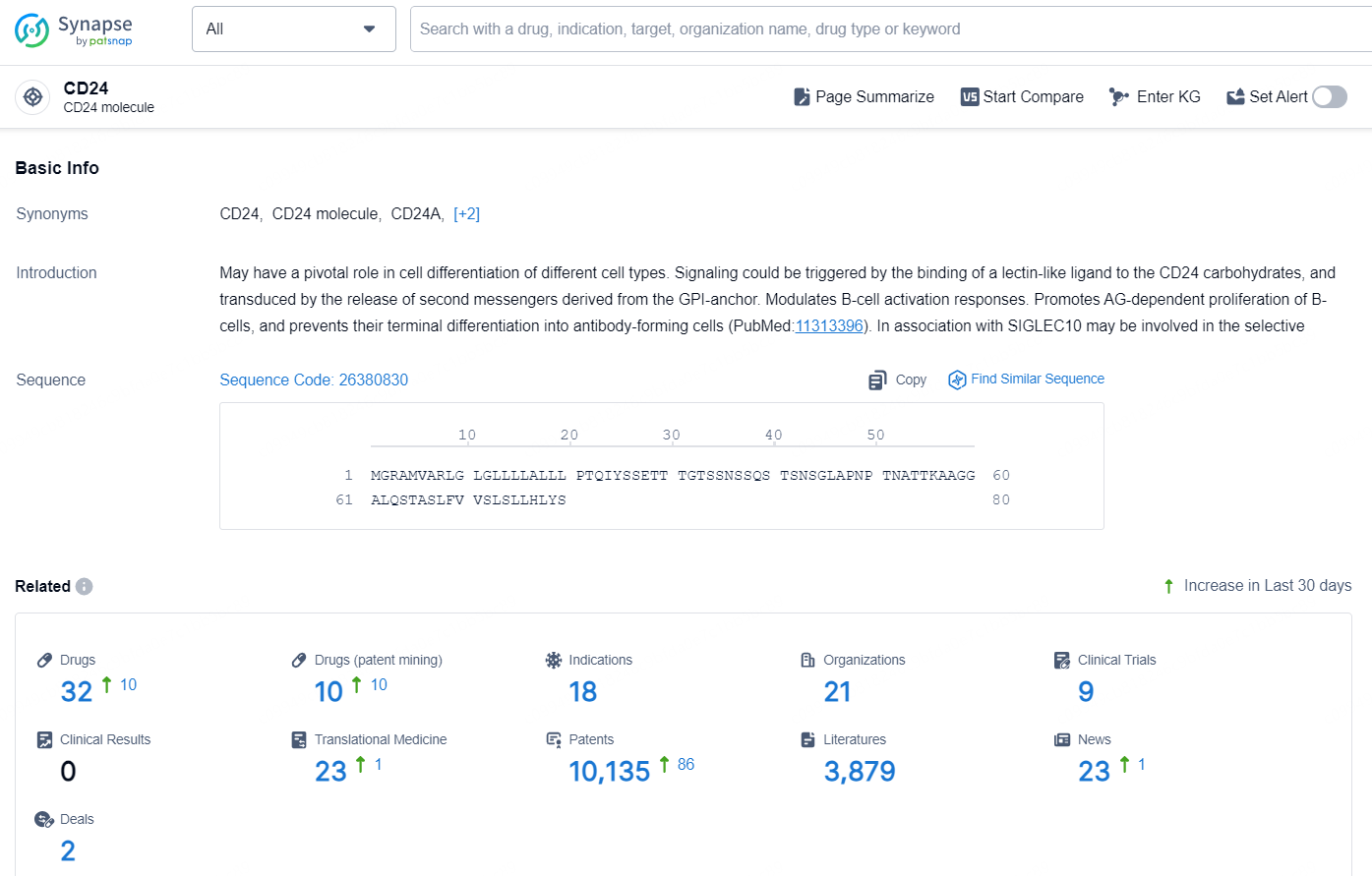
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Chemical, As of November 11, 2024, there are 32 investigational drugs for the CD24 target, including 18 indications, 21 R&D institutions involved, with related clinical trials reaching 9, and as many as 10135 patents.
PHST-001 is a monoclonal antibody drug developed by Pheast Therapeutics Inc. The drug targets CD24 and is intended for the treatment of neoplasms. As a monoclonal antibody, PHST-001 is designed to specifically bind to CD24, which is a protein found on the surface of cancer cells. This targeted approach may allow for the selective destruction of cancer cells while minimizing damage to healthy cells.