Takeda and Protagonist Sign Global Deal for Experimental Blood Disorder Drug, Rusfertide
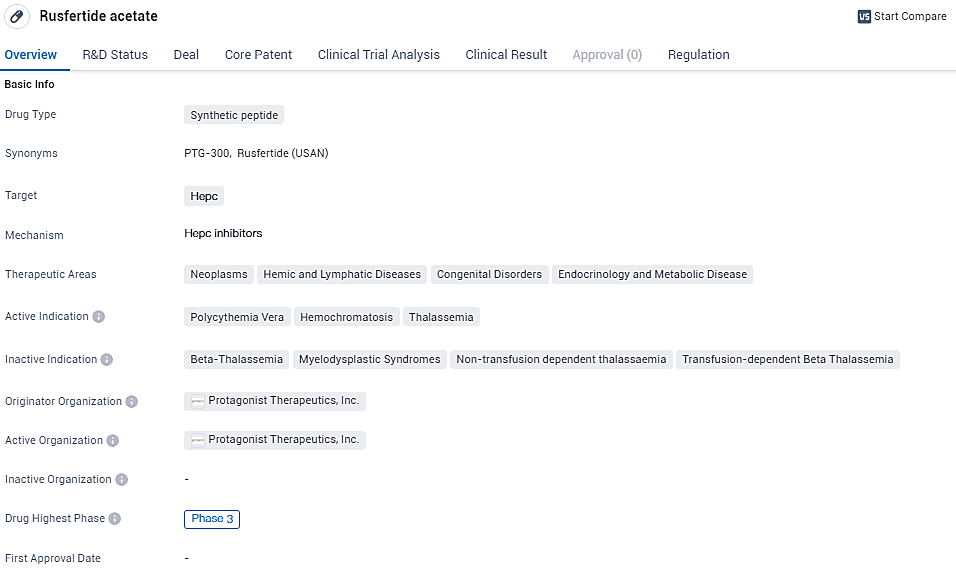
Takeda, in partnership with Protagonist Therapeutics, Inc., has declared the initiation of a global licensing and joint venture pact focusing on the advancement and marketing of an experimental drug known as rusfertide. This drug is a synthesized injectable peptide designed to imitate the action of the naturally occurring hormone hepcidin. Currently, rusfertide is undergoing a critical Phase 3 study named VERIFY, with the aim of evaluating its efficacy in treating the condition known as Polycythemia Vera.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Polycythemia Vera (PV) stands out as an uncommon, long-standing hematological condition that triggers an overproduction of red blood cells. It impacts up to 160,000 individuals within the United States, exhibiting a prevalence rate in Europe that echoes this figure. Crucial characteristics of PV include elevated levels of red blood cells coupled with a deficiency in iron, elevating the likelihood of patients encountering cardiovascular and thrombotic difficulties, including myocardial infarction and cerebral strokes. Such health challenges have a detrimental effect on patient well-being, leading to ailments like relentless exhaustion and mental disarray.
In accordance with the finalized collaborative arrangement, Protagonist is set to obtain an immediate disbursement of $300 million and could potentially acquire further compensations tied to worldwide developments, regulatory benchmarks, and tiered royalties dependent on non-U.S. sales proceeds. Moreover, Protagonist retains the duties of conducting research and overseeing development until the Phase 3 clinical trial concludes and approval is secured from U.S. regulatory bodies. Takeda, on the other hand, has secured rights regarding development outside the U.S. and is tasked with spearheading commercialization efforts on a global scale.
Dinesh V. Patel, Ph.D., the President and Chief Executive Officer of Protagonist Therapeutics, expressed his viewpoint: “As innovators in the realm of peptide therapeutics in pharmaceuticals, we are convinced that the synergy between our inventive capabilities and development expertise reaches its zenith when paired with an apt collaborator at the opportune moment.”
Patel added, “In our journey to evolve into a fully-fledged pharmaceutical entity, this strategic collaboration diminishes the intrinsic risks tied to embarking on our initial commercialization venture, strategically aligns the entry time, and maximizes our projected sales ceiling for rusfertide. Simultaneously, it offers us the chance to actively contribute to and benefit from the commercial success in the U.S. market with an evenly shared profit ratio.”
This newly declared partnership enhances Takeda's legacy in the specialized area of Rare Hematology. It comes on the heels of the recent endorsement by the U.S. Food and Drug Administration of ADZYNMA, Takeda's novel therapeutic intervention for congenital thrombotic thrombocytopenic purpura, a highly rare disorder associated with blood clots.
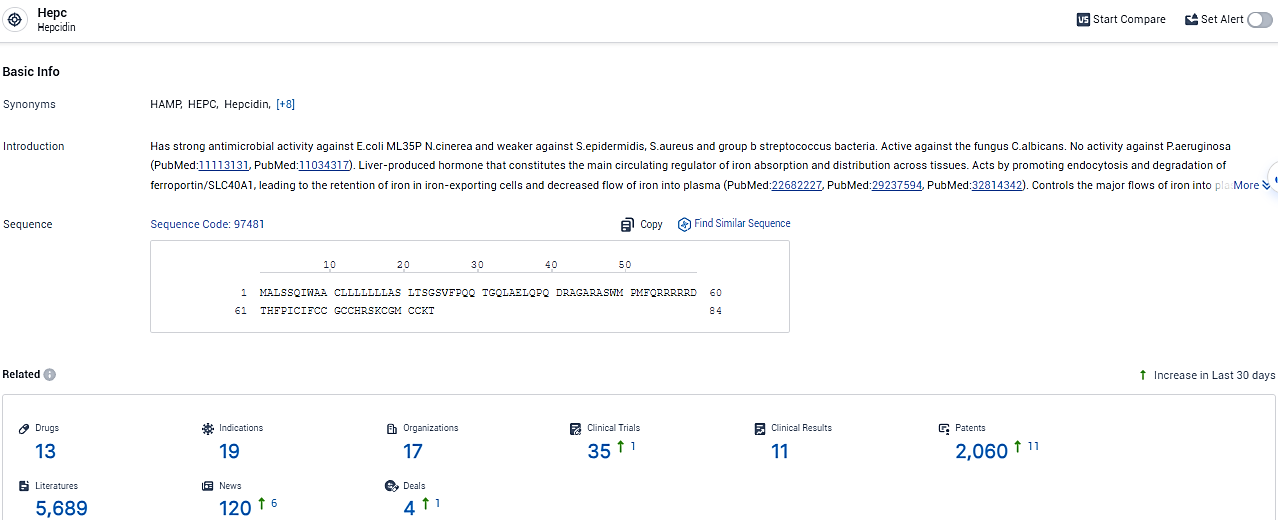
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of February 7, 2024, there are 13 investigational drugs for the Hepc target, including 19 indications, 17 R&D institutions involved, with related clinical trials reaching 35, and as many as 2060 patents.
Rusfertide shows potential as a treatment option for patients with Polycythemia Vera, Hemochromatosis, and Thalassemia. These conditions are characterized by abnormal blood cell production, iron overload, and abnormal hemoglobin production, respectively. By targeting Hepc, the drug may be able to address the underlying mechanisms of these diseases and provide therapeutic benefits.