Jaguar Gene Therapy's JAG201 Trials Approved by FDA for Autism and Phelan-McDermid Syndrome Treatment
Biotech firm Jaguar Gene Therapy, which is at the forefront of advancing gene therapy innovations for individuals grappling with serious genetic conditions, encompassing ailments impacting a significant number of patients, has recently declared that the U.S. Food and Drug Administration has given the green light for the firm's Investigational New Drug Application concerning JAG201. This gene therapy targets a specialized type of autism spectrum disorder alongside Phelan-McDermid syndrome.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
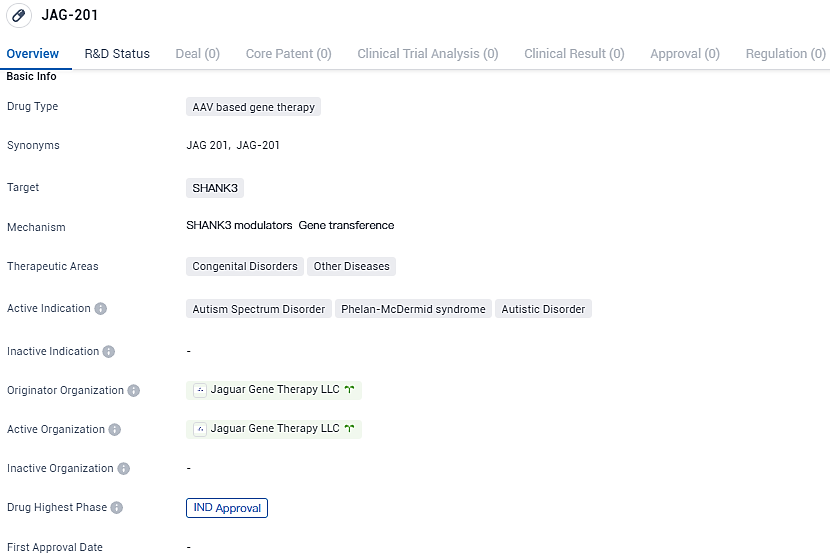
As of now, an estimated 30,000 individuals in the United States affected by ASD or PMS lack targeted therapies when their condition involves a SHANK3 gene aberration. JAG201 is being developed to supply functional SHANK3 through the deployment of the AAV9 vector to address the fundamental basis of the disorder.
Studies with preclinical models, including both rodents and primates that are not human, have shown that administering functional SHANK3 can enhance various neurological, cognitive, and motor skills that are typically atypical in these conditions. The developers intend to launch an initial Phase I clinical study for adult patients in the U.S. diagnosed with ASD or PMS due to SHANK3 mutations or deletions during the latter part of this year.
Jaguar Gene Therapy's CEO, Joe Nolan, expressed satisfaction with the FDA's approval to progress their SHANK3 gene therapy research into a clinical setting. Nolan remarked on the promising preclinical outcomes that suggest JAG201 could be groundbreaking for those impacted by the syndrome. He emphasized the importance of this advancement not just for Jaguar Gene Therapy but predominantly for those around 30,000 people dealing with genetic-linked autism or Phelan-McDermid syndrome.
These individuals confront a spectrum of symptoms, including significant developmental delays, challenges in speech and communication, and progressive social, cognitive, and motor skills decline, often needing round-the-clock care. There are no existing therapeutic strategies that confront the core issue, and Nolan stated the commitment to make a difference in this area.
Ronni Blumenthal, CEO of the Phelan-McDermid Syndrome Foundation, also shared her enthusiasm regarding the FDA's green light for JAG201's clinical trials. She pointed out the dire need within the community for novel therapies capable of mitigating or even eliminating some of the more severe effects of the condition, thereby potentially enhancing the quality of life for their loved ones.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
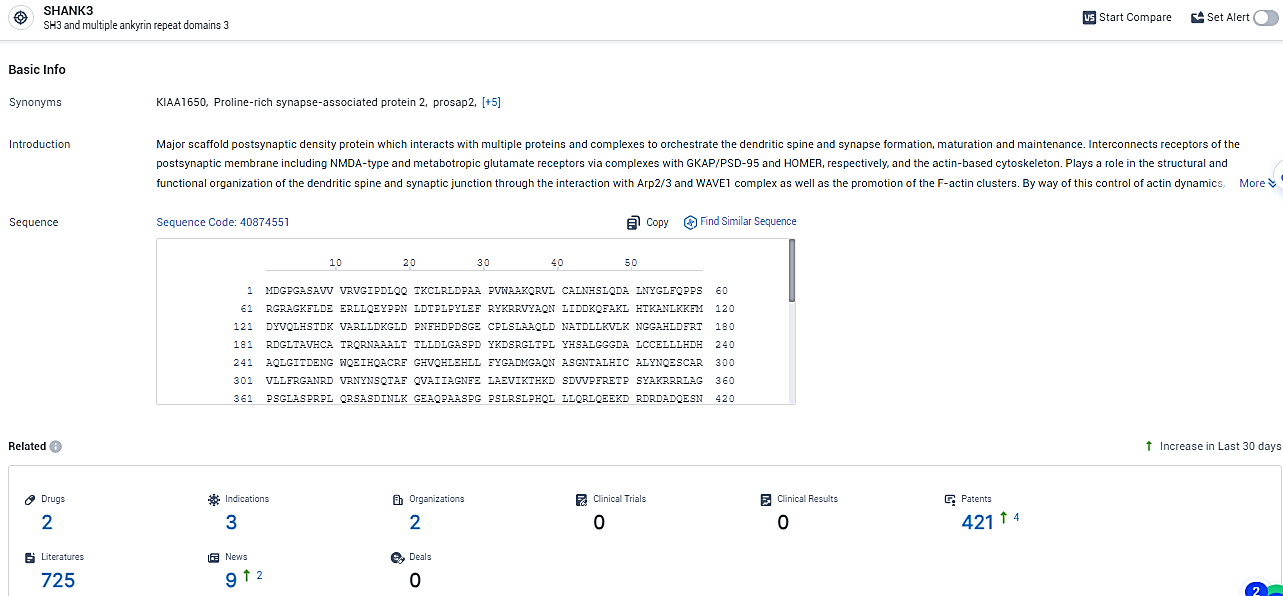
According to the data provided by the Synapse Database, As of February 7, 2024, there are 2 investigational drugs for the SHANK3 target, including 3 indications, 2 R&D institutions involved, and as many as 421 patents.
JAG-201 aims to address the underlying genetic causes of Autism Spectrum Disorder, Phelan-McDermid syndrome, and Autistic Disorder. With IND approval, JAG-201 is poised to enter clinical trials, potentially offering a novel therapeutic approach for individuals affected by these disorders.